Themed collection Lignin chemistry and valorisation

Unlocking the potential of a sleeping giant: lignins as sustainable raw materials for renewable fuels, chemicals and materials
Editorial article to accompany the Green Chemistry themed issue ‘Lignin Chemistry and Valorisation’.

Green Chem., 2015,17, 4860-4861
https://doi.org/10.1039/C5GC90055G
Thermochemical conversion of lignin to functional materials: a review and future directions
The naturally abundant lignin offers a sustainable platform for the synthesis of functional carbon materials which have been widely used in catalysis, energy storage, and pollutant removal.
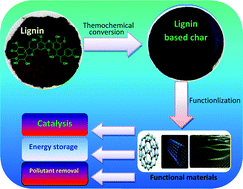
Green Chem., 2015,17, 4888-4907
https://doi.org/10.1039/C5GC01054C
Thermal properties of lignin in copolymers, blends, and composites: a review
Modulating thermal properties via lignin copolymers, blends, and composites.
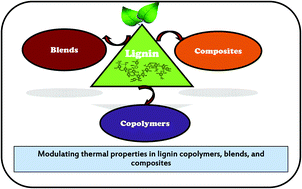
Green Chem., 2015,17, 4862-4887
https://doi.org/10.1039/C5GC01066G
Fractionation of lignin from eucalyptus bark using amine-sulfonate functionalized ionic liquids
Amine-sulfonate functionalized ionic liquids not only dissolve industrial lignin materials like kraft lignin and lignosulfonate, but also offer unique selectivity and efficiency in fractionating lignin from eucalyptus bark.
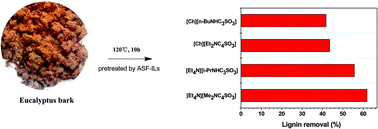
Green Chem., 2015,17, 4913-4920
https://doi.org/10.1039/C5GC01035G
Base-catalysed cleavage of lignin β-O-4 model compounds in dimethyl carbonate
Dimethyl carbonate (DMC) was used as solvent and non-toxic capping agent in a base-catalysed selective cleavage of lignin model compounds.
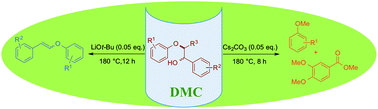
Green Chem., 2015,17, 4908-4912
https://doi.org/10.1039/C5GC00186B
Mechanistic investigation of the Zn/Pd/C catalyzed cleavage and hydrodeoxygenation of lignin
While current biorefinery processes use lignin only for its heat value, the conversion of lignin to high value chemicals is an area of increasing interest.
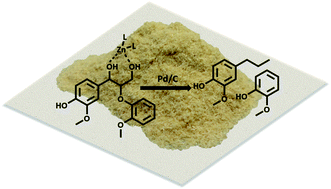
Green Chem., 2016,18, 2399-2405
https://doi.org/10.1039/C5GC01325A
Hydrolytic liquefaction of hydrolysis lignin for the preparation of bio-based rigid polyurethane foam
Hydrolysis lignin (HL) was liquefied employing 50/50 (v/v) water–ethanol mixture for the preparation of bio-based polyols/rigid polyurethane foams.
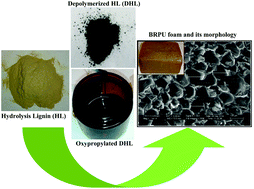
Green Chem., 2016,18, 2385-2398
https://doi.org/10.1039/C5GC02876K
Reductive deconstruction of organosolv lignin catalyzed by zeolite supported nickel nanoparticles
Breaking good: In situ ATR-IR spectroscopy tracked the Ni catalyzed deconstruction and hydrodeoxygenation of organosolv lignin in n-hexadecane, towards the production of naphthenes.
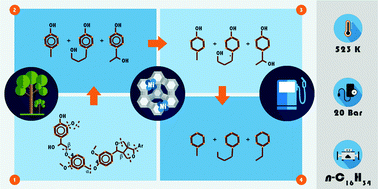
Green Chem., 2015,17, 5079-5090
https://doi.org/10.1039/C5GC02160J
Path to plastics composed of ligninsulphonates (lignosulfonates)
In tensile behaviour, polymeric materials containing only methylated ball-milled lignin surpass polystyrene, while 85% w/w ligninsulphonate blends approach polyethylene.
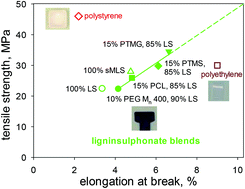
Green Chem., 2015,17, 5069-5078
https://doi.org/10.1039/C5GC01865J
Lignin isolation from spruce wood with low concentration aqueous alkali at high temperature and pressure: influence of hot-water pre-extraction
Lignin was successfully isolated from spruce wood with an accelerated solvent extractor using low concentration aqueous NaOH as an extraction solvent. The biorefinery concept was also applied.
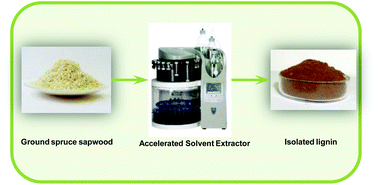
Green Chem., 2015,17, 5058-5068
https://doi.org/10.1039/C5GC01341K
Influence of bio-based solvents on the catalytic reductive fractionation of birch wood
In the reductive catalytic fractionation of lignocellulose, the choice of solvent significantly impacts the delignification efficiency, carbohydrate retention in the pulp and the macrostructure of the pulp.

Green Chem., 2015,17, 5035-5045
https://doi.org/10.1039/C5GC01442E
Structural changes in lignins isolated using an acidic ionic liquid water mixture
Recently, acidic ionic liquid water mixtures based on the hydrogen sulfate anion have been shown to effectively extract lignin from lignocellulosic biomass.
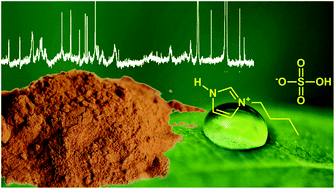
Green Chem., 2015,17, 5019-5034
https://doi.org/10.1039/C5GC01314C
Efficient catalytic hydrotreatment of Kraft lignin to alkylphenolics using supported NiW and NiMo catalysts in supercritical methanol
Efficient catalytic hydrotreatment of Kraft lignin to yield aromatic monomers was demonstrated in supercritical methanol using a variety of NiW and NiMo catalysts on acidic, basic and neutral supports.
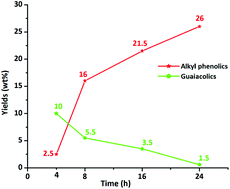
Green Chem., 2015,17, 5046-5057
https://doi.org/10.1039/C5GC01643F
Oxidative conversion of lignin and lignin model compounds catalyzed by CeO2-supported Pd nanoparticles
Pd/CeO2 efficiently catalyzes the oxidative conversion of 2-phenoxy-1-phenylethanol, a lignin model compound with a β-O-4 linkage, in methanol, producing monomeric aromatics.
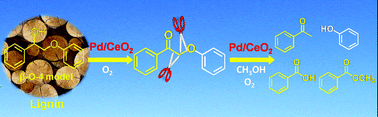
Green Chem., 2015,17, 5009-5018
https://doi.org/10.1039/C5GC01473E
Iron-catalysed oxidative cleavage of lignin and β-O-4 lignin model compounds with peroxides in DMSO
Simple FeCl3-derived iron catalysts are used for the cleavage of lignin and β-O-4 lignin model compounds.

Green Chem., 2015,17, 5001-5008
https://doi.org/10.1039/C5GC01306B
Solvent free depolymerization of Kraft lignin to alkyl-phenolics using supported NiMo and CoMo catalysts
The catalytic hydrotreatment of Kraft lignin using sulfided NiMo and CoMo catalysts on different acidic and basic supports (Al2O3, ZSM-5, activated carbon (AC) and MgO-La2O3) was studied in the absence of a solvent.
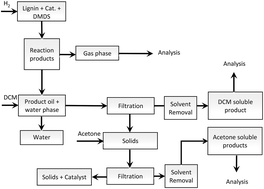
Green Chem., 2015,17, 4921-4930
https://doi.org/10.1039/C5GC01641J
The synthesis and analysis of advanced lignin model polymers
Advanced lignin model polymers have been synthesised to aid in the development of new methods for lignin valorisation.
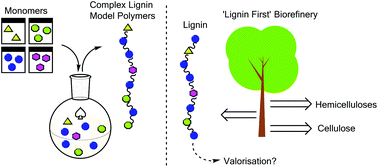
Green Chem., 2015,17, 4980-4990
https://doi.org/10.1039/C5GC01334H
Reversible crosslinking of lignin via the furan–maleimide Diels–Alder reaction
Lignoboost lignin was independently functionalized with furan and maleimide groups. Taken together, these functionalized lignins show all the features of reversible networks.
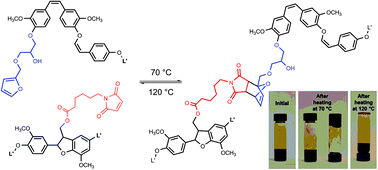
Green Chem., 2015,17, 4991-5000
https://doi.org/10.1039/C5GC01319D
Exploring glutathione lyases as biocatalysts: paving the way for enzymatic lignin depolymerization and future stereoselective applications
One pot – two enzymes. The combination of β-etherases with glutathione lyases enables the selective cleavage of lignin model compounds. Protein engineering of glutathione lyases allows modification of activity and stereoselectivity.
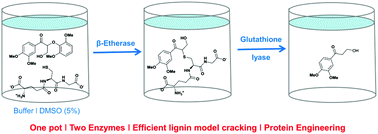
Green Chem., 2015,17, 4931-4940
https://doi.org/10.1039/C5GC01078K
Biocatalytic conversion of lignin to aromatic dicarboxylic acids in Rhodococcus jostii RHA1 by re-routing aromatic degradation pathways
A gene insertion approach is used in Rhodococcus jostii RHA1 to generate pyridine-dicarboxylic acid bioproducts from lignin.
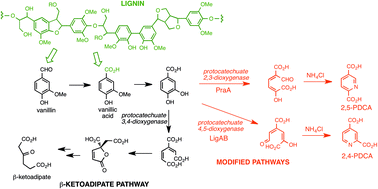
Green Chem., 2015,17, 4974-4979
https://doi.org/10.1039/C5GC01347J
Selective oxidative C–C bond cleavage of a lignin model compound in the presence of acetic acid with a vanadium catalyst
The solvent has great effects on vanadium-catalyzed oxidative C–C bond cleavage of 2-phenoxy-1-phenylethanol with molecular oxygen.
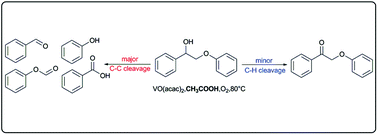
Green Chem., 2015,17, 4968-4973
https://doi.org/10.1039/C5GC00645G
Ethanol as capping agent and formaldehyde scavenger for efficient depolymerization of lignin to aromatics
High monomer yield (60–86 wt%) with little char formation is possible from lignin. Ethanol acts as capping agent of aromatics, as formaldehyde scavenger and hydrogen source, prevent repolymerization.
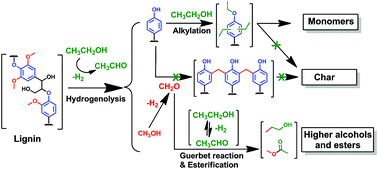
Green Chem., 2015,17, 4941-4950
https://doi.org/10.1039/C5GC01120E
Towards lignin consolidated bioprocessing: simultaneous lignin depolymerization and product generation by bacteria
Lignin Consolidated Bioprocessing utilizes microbes that simultaneously depolymerize lignin and convert the resulting aromatic compounds to fuel and chemical precursors.
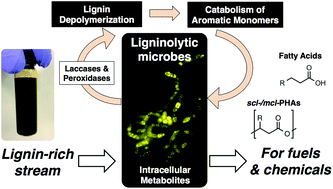
Green Chem., 2015,17, 4951-4967
https://doi.org/10.1039/C5GC01165E
About this collection
Efficient production of biobased chemicals, materials and fuels requires the valorisation of lignin, a biopolymer that comprises up to one-third of the composition and one-fourth of the energy content of lignocellulose. The structural complexity and general recalcitrance of this aromatic biopolymer pose considerable challenges to the elucidation and modification of its structure in addition to its valorisation to green products.
Recently, the scientific community has made significant progress in engineering lignin, characterising its structural features, valorising the biopolymer through catalysis, and finding new outlets for the lignin-derived products.
This themed issue highlights the many aspects of lignin research, the advances made in valorisation and the potential of this abundant and critically important feed component of future biorefineries. The guest editors for this themed issue are Professor Bert Weckhuysen (Utrecht University, Netherlands), Dr Pieter Bruijnincx (Utrecht University, Netherlands), and Dr Roberto Rinaldi (Imperial College London, UK).