Themed collection Molecular Design for Reduced Toxicity

Safer by Design
Editorial to accompany the part-themed issue on molecular design for reduced toxicity.

Green Chem., 2016,18, 4324-4324
https://doi.org/10.1039/C6GC90074G
Alarms about structural alerts
Integrative approach for safety assessment of new chemicals by combining structural alerts and QSAR models.
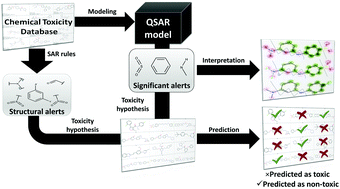
Green Chem., 2016,18, 4348-4360
https://doi.org/10.1039/C6GC01492E
On the design of safer chemicals: a path forward
A collaborative effort must be made by government agencies, industry and academia to develop safer commercial chemicals.

Green Chem., 2016,18, 4332-4347
https://doi.org/10.1039/C6GC00526H
Connecting toxicology and chemistry to ensure safer chemical design
Designing safer, healthier and sustainable products and processes requires the engagement of toxicologists and the incorporation of twenty-first century toxicology principles and practices.

Green Chem., 2016,18, 4325-4331
https://doi.org/10.1039/C6GC00758A
Zebrafish as an in vivo model for sustainable chemical design
Heightened public awareness about the many thousands of chemicals in use and present as persistent contaminants in the environment has increased the demand for safer chemicals and more rigorous toxicity testing.
Green Chem., 2016,18, 6410-6430
https://doi.org/10.1039/C6GC02061E
QSAR models of human data can enrich or replace LLNA testing for human skin sensitization
An example of structural transformation of human skin sensitizers into various non-sensitizers based on interpretation of QSAR models.
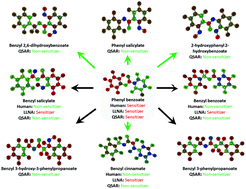
Green Chem., 2016,18, 6501-6515
https://doi.org/10.1039/C6GC01836J
Predicting skin permeation rate from nuclear magnetic resonance spectra
A predictive method is reported for estimating skin permeation of organic chemicals exclusively from NMR spectroscopic data and molecular weight, which does not require knowledge of chemical structure.
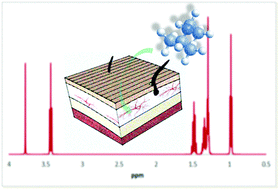
Green Chem., 2016,18, 4468-4474
https://doi.org/10.1039/C6GC00945J
Probabilistic diagram for designing chemicals with reduced potency to incur cytotoxicity
A probabilistic diagram presenting the complete solution in the variable space to guide safer chemical design against cytotoxicity.
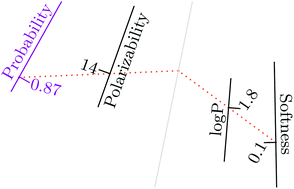
Green Chem., 2016,18, 4461-4467
https://doi.org/10.1039/C6GC01058J
On the way to greener ionic liquids: identification of a fully mineralizable phenylalanine-based ionic liquid
Biodegradability screening of L-phenylalanine based ILs and LC-HRMS analysis of the degradation products led to the identification of a pyridinium IL which was fully mineralizable and is proposed as a basic structure for green ILs.
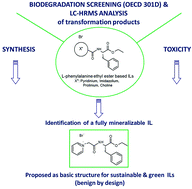
Green Chem., 2016,18, 4361-4373
https://doi.org/10.1039/C6GC00417B
Synthesis of a series of amino acid derived ionic liquids and tertiary amines: green chemistry metrics including microbial toxicity and preliminary biodegradation data analysis
A series of L-phenylalanine and L-tyrosine derived ionic liquids and tertiary amines were evaluated according to principles of green chemistry.
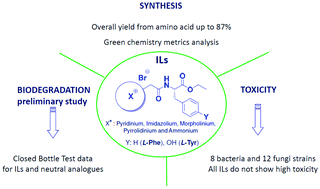
Green Chem., 2016,18, 4374-4392
https://doi.org/10.1039/C6GC00415F
Alkyl melibioside and alkyl cellobioside surfactants: effect of sugar headgroup and alkyl chain length on performance
The potential of glycolipid surfactants, composed of a sugar headgroup and lipid tail, as highly biodegradable and less toxic alternatives to commonly used surfactants motivates the systematic study of structure–function relationships of various glycolipid surfactants.
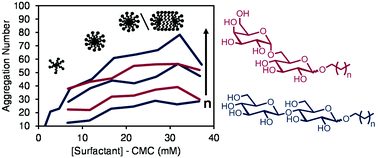
Green Chem., 2016,18, 4446-4460
https://doi.org/10.1039/C6GC00436A
The ecotoxicogenomic assessment of soil toxicity associated with the production chain of 2,5-furandicarboxylic acid (FDCA), a candidate bio-based green chemical building block
2,5-Furan dicarboxylic acid (FDCA) is one of the top-12 value-added chemicals derived from biomass that may serve as a ‘green’ substitute for terephthalic acid (TPA) in polyesters.
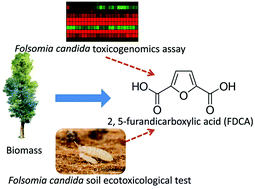
Green Chem., 2016,18, 4420-4431
https://doi.org/10.1039/C6GC00430J
Assessment of predictive models for estimating the acute aquatic toxicity of organic chemicals
In silico toxicity models are critical in addressing experimental aquatic toxicity data gaps and prioritizing chemicals for further assessment.
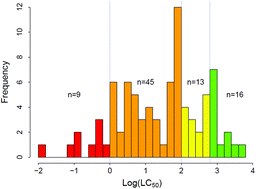
Green Chem., 2016,18, 4432-4445
https://doi.org/10.1039/C6GC00720A
A chemical–biological similarity-based grouping of complex substances as a prototype approach for evaluating chemical alternatives
An experimental and computational approach to categorizing UVCBs according to chemical and biological similarities.
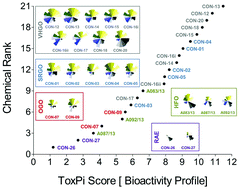
Green Chem., 2016,18, 4407-4419
https://doi.org/10.1039/C6GC01147K
Aquatic ecotoxicity of personal care products: QSAR models and ranking for prioritization and safer alternatives’ design
New externally validated QSAR models for aquatic toxicity of PCPs are proposed and applicable in QSARINS for the a priori chemical design of environmentally safer PCPs.
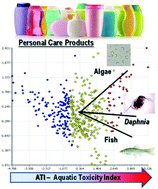
Green Chem., 2016,18, 4393-4406
https://doi.org/10.1039/C5GC02818C
About this collection
Molecular design for reduced toxicity is an area of green chemistry which seeks to increase our understanding and enable the design of molecules across structural classes and applications that can provide the required functions, without the undesirable toxicity. This themed issue, guest-edited by Paul Anastas, Julie Zimmerman and Adelina Voutchkova-Kostal explores the advances being made in this emerging field.