Themed collection Electrocatalysis - Fundamental Insights for Sustainable Energy

Electrocatalysis
This themed issue presents a collection of articles on electrocatalysis.
Phys. Chem. Chem. Phys., 2014,16, 13567-13567
https://doi.org/10.1039/C4CP90068E
Electrocatalysis on gold
This perspective article reviews recent advances in the study of important catalytic reactions on gold electrodes.
Phys. Chem. Chem. Phys., 2014,16, 13583-13594
https://doi.org/10.1039/C4CP00394B
Nanostructured carbon-based cathode catalysts for nonaqueous lithium–oxygen batteries
Structure–activity correlations of nanocarbon oxygen cathode catalysts for nonaqueous Li–O2 batteries are discussed, providing guidance for rational catalyst design.
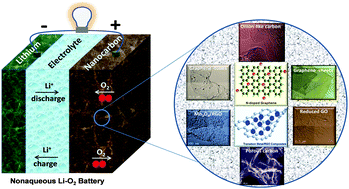
Phys. Chem. Chem. Phys., 2014,16, 13568-13582
https://doi.org/10.1039/C4CP00225C
Alkaline O2 reduction on oxide-derived Au: high activity and 4e− selectivity without (100) facets
Despite having no detectable (100) facets, oxide-derived Au electrodes have 4e− O2 reduction activity that rivals (100)-rich Au cubes.
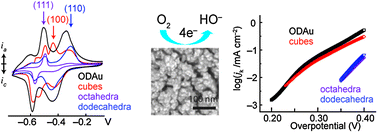
Phys. Chem. Chem. Phys., 2014,16, 13601-13604
https://doi.org/10.1039/C4CP01337A
Engineering self-assembled N-doped graphene–carbon nanotube composites towards efficient oxygen reduction electrocatalysts
Tuning the composition of N-doped graphene–carbon nanotube composites can boost their electrocatalytic performance for the oxygen reduction reaction.

Phys. Chem. Chem. Phys., 2014,16, 13605-13609
https://doi.org/10.1039/C4CP00757C
Electrocatalysis of hydrogen peroxide reactions on perovskite oxides: experiment versus kinetic modeling
A kinetic model sheds light on the mechanism of the hydrogen peroxide reactions on Mn- and Co-perovskite oxides.
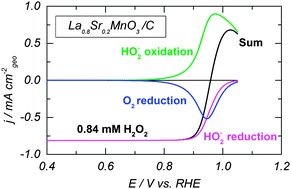
Phys. Chem. Chem. Phys., 2014,16, 13595-13600
https://doi.org/10.1039/C4CP00341A
Insights into the electrocatalytic reduction of CO2 on metallic silver surfaces
The potential dependent activity and selectivity of CO2 electroreduction on polycrystalline silver surfaces are investigated, providing insights into mechanistic aspects, including C–C coupling.
Phys. Chem. Chem. Phys., 2014,16, 13814-13819
https://doi.org/10.1039/C4CP00692E
A density functional theory study of oxygen reduction reaction on non-PGM Fe–Nx–C electrocatalysts
First-principles density functional theory (DFT) calculations were performed to explain the stability of catalytically active sites in Fe–Nx–C electrocatalysts, their ORR activity and ORR mechanism.
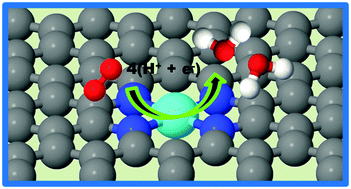
Phys. Chem. Chem. Phys., 2014,16, 13800-13806
https://doi.org/10.1039/C4CP01634C
The influence of reactive side products on the electrooxidation of methanol – a combined in situ infrared spectroscopy and online mass spectrometry study
The impact of formaldehyde and formic acid on the electrooxidation of methanol is studied by in situ infrared spectroscopy and online mass spectrometry revealing a non-additive behavior.
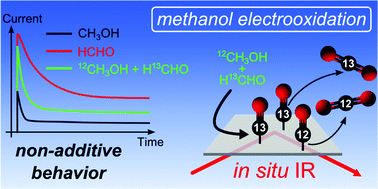
Phys. Chem. Chem. Phys., 2014,16, 13780-13799
https://doi.org/10.1039/C4CP01229A
Electrocatalytic activity of various types of h-BN for the oxygen reduction reaction
Enhancement of oxygen reduction reaction activity of a Au electrode by modification with various types of BN nanostructure.
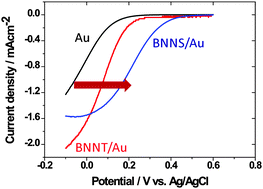
Phys. Chem. Chem. Phys., 2014,16, 13755-13761
https://doi.org/10.1039/C4CP00402G
The influence of a fibrous carbon envelope on the formation of CoFe nanoparticles for durable electrocatalytic oxygen evolution
Non-precious metal electrocatalyst: CoFe particles surrounded by fibrous carbon for the elongated stability of oxygen evolution from water molecules.
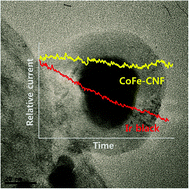
Phys. Chem. Chem. Phys., 2014,16, 13807-13813
https://doi.org/10.1039/C4CP00385C
Towards the elucidation of the high oxygen electroreduction activity of PtxY: surface science and electrochemical studies of Y/Pt(111)
Insight into the high oxygen electroreduction activity of PtxY by studying single crystal Y/Pt(111).
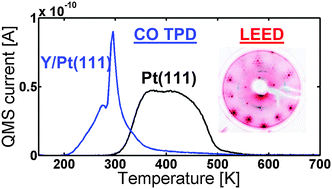
Phys. Chem. Chem. Phys., 2014,16, 13718-13725
https://doi.org/10.1039/C4CP00319E
Effect of ordering of PtCu3 nanoparticle structure on the activity and stability for the oxygen reduction reaction
The performance enhancement effect of structural ordering for the oxygen reduction reaction (ORR) on the PtCu3 nanoparticulate system is systematically studied.
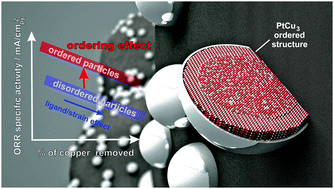
Phys. Chem. Chem. Phys., 2014,16, 13610-13615
https://doi.org/10.1039/C4CP00585F
In situ back-side illumination fluorescence XAFS (BI-FXAFS) studies on platinum nanoparticles deposited on a HOPG surface as a model fuel cell: a new approach to the Pt-HOPG electrode/electrolyte interface
We measured the in situ polarization-dependent X-ray absorption fine structure of PtNPs deposited on a flat HOPG substrate.
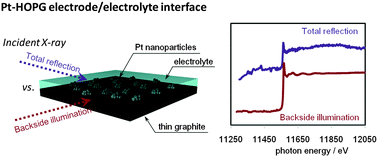
Phys. Chem. Chem. Phys., 2014,16, 13748-13754
https://doi.org/10.1039/C4CP00265B
Alkali cation specific adsorption onto fcc(111) transition metal electrodes
Density functional theory suggests alkali metal specific adsorption may compete with hydrogen on some metal electrodes in alkaline electrolytes.
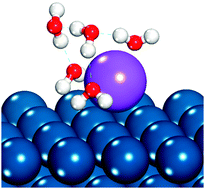
Phys. Chem. Chem. Phys., 2014,16, 13699-13707
https://doi.org/10.1039/C4CP00760C
Density functional theory study of carbon dioxide electrochemical reduction on the Fe(100) surface
The CO2 electroreduction mechanism and electrode stability on Fe(100) electrocatalysts is examined with density functional theory.
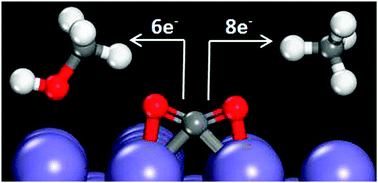
Phys. Chem. Chem. Phys., 2014,16, 13708-13717
https://doi.org/10.1039/C4CP00266K
Tuning the oxygen reduction activity of the Pt–Ni nanoparticles upon specific anion adsorption by varying heat treatment atmospheres
A proper heat treatment atmosphere decisively influenced the oxygen reduction reaction, particularly upon specific anion adsorption from the half-cell to single-cell scale.

Phys. Chem. Chem. Phys., 2014,16, 13726-13732
https://doi.org/10.1039/C4CP00187G
On the faradaic selectivity and the role of surface inhomogeneity during the chlorine evolution reaction on ternary Ti–Ru–Ir mixed metal oxide electrocatalysts
Faradaic selectivity of the chlorine and oxygen evolution (left) is linked to the spatial inhomogeneity of the surface reactivity of Ti–Ru–Ir mixed metal oxide catalysts.

Phys. Chem. Chem. Phys., 2014,16, 13741-13747
https://doi.org/10.1039/C4CP00896K
Structural effects on the oxygen reduction reaction on the high index planes of Pt3Co
Structural effects on the activity for the oxygen reduction reaction on the high index planes of Pt3Co.
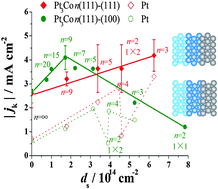
Phys. Chem. Chem. Phys., 2014,16, 13774-13779
https://doi.org/10.1039/C4CP00243A
Specific adsorption of perchlorate anions on Pt{hkl} single crystal electrodes
Perchlorate anion adsorption inhibits the oxygen reduction reaction on Pt{hkl} electrodes in aqueous perchloric acid due to weak specific adsorption.
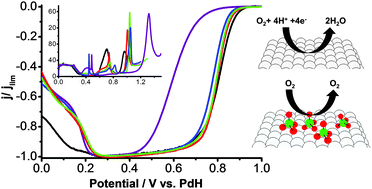
Phys. Chem. Chem. Phys., 2014,16, 13689-13698
https://doi.org/10.1039/C4CP00564C
Mechanistic and kinetic implications on the ORR on a Au(100) electrode: pH, temperature and H–D kinetic isotope effects
The potential determining reaction and the rate-determining step for the ORR on Au(100) in acidic and alkaline solution have been clarified.
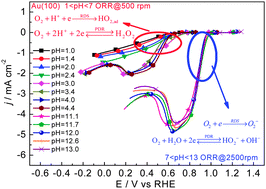
Phys. Chem. Chem. Phys., 2014,16, 13762-13773
https://doi.org/10.1039/C4CP00257A
A density functional theory study of catalytic sites for oxygen reduction in Fe/N/C catalysts used in H2/O2 fuel cells
The oxygen reduction catalytic activity of carbon-supported FeN4 moieties bridging micropores between two graphene sheets was investigated by density functional theory (DFT).
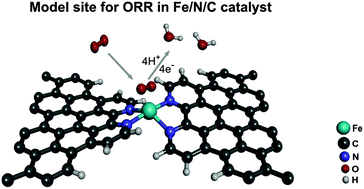
Phys. Chem. Chem. Phys., 2014,16, 13654-13661
https://doi.org/10.1039/C3CP55331K
Following ORR intermediates adsorbed on a Pt cathode catalyst during break-in of a PEM fuel cell by in operando X-ray absorption spectroscopy
In operando X-ray absorption spectroscopy data using the Δμ X-ray Absorption Near Edge Spectroscopy (XANES) analysis procedure is used to follow the ORR intermediate adsorbate coverage on a working catalyst in a PEMFC during initial activation and break-in.
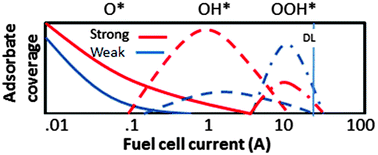
Phys. Chem. Chem. Phys., 2014,16, 13645-13653
https://doi.org/10.1039/C4CP00192C
Terpyridine complexes of first row transition metals and electrochemical reduction of CO2 to CO
Homoleptic terpyridine complexes of 3d transition metals are found to electrocatalytically reduce CO2 to either CO or tuneable CO–H2 mixtures.
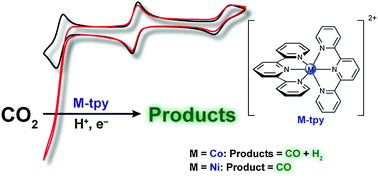
Phys. Chem. Chem. Phys., 2014,16, 13635-13644
https://doi.org/10.1039/C4CP00451E
Elucidating the activity of stepped Pt single crystals for oxygen reduction
The activity of stepped Pt can be described by a Sabatier type volcano, according to a voltammetric analysis.
Phys. Chem. Chem. Phys., 2014,16, 13625-13629
https://doi.org/10.1039/C4CP00260A
Beyond the volcano limitations in electrocatalysis – oxygen evolution reaction
Oxygen evolution catalysis is restricted by the interdependence of adsorption energies of the reaction intermediates and the surface reactivity.

Phys. Chem. Chem. Phys., 2014,16, 13682-13688
https://doi.org/10.1039/C4CP00571F
Pt nanoparticles supported on Sb-doped SnO2 porous structures: developments and issues
Control of the metal oxide surface properties leads in the case of Sb–SnO2 to a support material for Pt nanoparticles with tailored catalyst corrosion stability and activity.

Phys. Chem. Chem. Phys., 2014,16, 13672-13681
https://doi.org/10.1039/C4CP00238E
Electrocatalytic oxygen reduction kinetics on Fe-center of nitrogen-doped graphene
OOH dissociation is the key step in electrocatalytic oxygen reduction on Fe–N centers of graphite, as revealed from first principles.

Phys. Chem. Chem. Phys., 2014,16, 13733-13740
https://doi.org/10.1039/C4CP00037D
Formic acid electrooxidation on thallium-decorated shape-controlled platinum nanoparticles: an improvement in electrocatalytic activity
Thallium deposited on Pt nanoparticles catalyzes formic acid electrooxidation.

Phys. Chem. Chem. Phys., 2014,16, 13616-13624
https://doi.org/10.1039/C4CP00304G
Halide adsorption on close-packed metal electrodes
In this study, we provide a complete picture of how adatoms can reduce the work function of a metal surface by contrasting two independent mechanisms.

Phys. Chem. Chem. Phys., 2014,16, 13630-13634
https://doi.org/10.1039/C4CP00237G
High activity of cubic PtRh alloys supported on graphene towards ethanol electrooxidation
Cubic PtRh alloys supported on graphene (PtxRhy/GN) possess enhanced activity and high selectivity for ethanol electrooxidation.
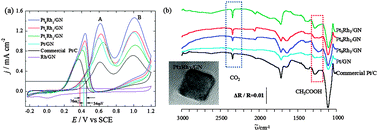
Phys. Chem. Chem. Phys., 2014,16, 13662-13671
https://doi.org/10.1039/C3CP55059A
Electronic modification of Pt via Ti and Se as tolerant cathodes in air-breathing methanol microfluidic fuel cells
We reported herein on the use of novel tolerant cathode catalysts: PtxTiy and/or PtxSey. Their prospective use as nanomaterials in an air-breathing methanol microfluidic fuel cells (laminar flow, and mixed reactant mode) is demonstrated.
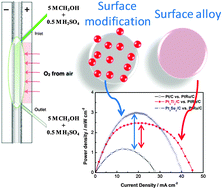
Phys. Chem. Chem. Phys., 2014,16, 13820-13826
https://doi.org/10.1039/C3CP54564D
About this collection
Electrocatalysis deals with the catalysis of redox reactions and is a key discipline for the development for energy storage, fuel cells, solar fuels, and all other electrochemical devices, in which the control of interfacial charge transfer reactions plays an important role. With the projected future “electrification” of our society through solar and wind power, the conversion of electricity to chemical bonds and vice versa will be an essential field of physical chemistry in the decades to come. Basic fundamental understanding of these processes, both in model studies and at a more device level, requires detailed quantitative physical-chemistry approaches. Therefore, an issue of PCCP dealing with a physical chemistry approach to electrocatalysis, balancing fundamental and more applied studies, is timely, and will have a significant impact on the future directions of this important field.