Themed collection Fluorine Chemistry

Fluorine-18: an untapped resource in inorganic chemistry
Advances in the field of fluorine chemistry have been applied extensively to the syntheses of 18F-labelled organic compounds and radiopharmaceuticals.
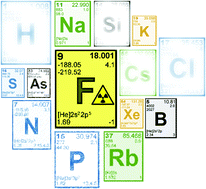
Chem. Commun., 2018,54, 11835-11842
https://doi.org/10.1039/C8CC04751K
Use of inorganic fluorinated materials in lithium batteries and in energy conversion systems
CFx-based materials bring significant improvement in terms of gravimetric and volumetric energy densities with respect to other types of primary Li batteries.
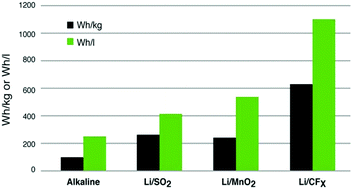
Chem. Commun., 2018,54, 11375-11382
https://doi.org/10.1039/C8CC05549A
Activation of C–F bonds α to C–C multiple bonds
A closer look is given to the successful approaches to the C(sp3)–F activation of benzylic, allylic, propargylic and allenylic fluorides.

Chem. Commun., 2018,54, 10224-10239
https://doi.org/10.1039/C8CC05108A
Recent advances in the synthesis of functionalised monofluorinated compounds
Over the past few years, we have tackled the synthesis of interesting monofluorinated organic molecules, such as: dihydronaphthalene derivatives, β-fluoro sulfones and related carbonyl compounds, fluorohydrins and allylic alcohols.
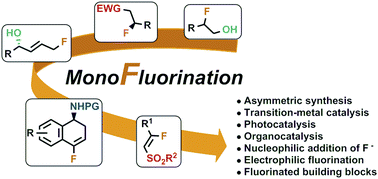
Chem. Commun., 2018,54, 9706-9725
https://doi.org/10.1039/C8CC05181J
Silicon-fluorine chemistry: from the preparation of SiF2 to C–F bond activation using silylenes and its heavier congeners
The feisty nature of silicon(II) fluorides has been harnessed by two cyclic alkyl amino carbene (cAAC) ligands and (cAAC)2SiF2 has been isolated at room temperature and structurally characterized.
Chem. Commun., 2018,54, 5046-5057
https://doi.org/10.1039/C8CC01816B
Fluorination of organoboron compounds
This review presents the methods available for the fluorination and radiofluorination of aromatic and aliphatic organoboron compounds.

Org. Biomol. Chem., 2019,17, 5651-5660
https://doi.org/10.1039/C9OB00832B
Recent progress in photochemical radical di- and mono-fluoromethylation
Di- and mono-fluoromethyl radicals can be easily accessed by photochemical protocols.
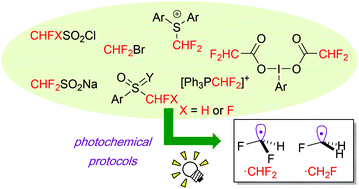
Org. Biomol. Chem., 2019,17, 5413-5419
https://doi.org/10.1039/C9OB00734B
Chemistry of detrifluoroacetylatively in situ generated fluoro-enolates
This review article provides a summary of the detrifluoroacetylative in situ generation of fluorine-containing enolates and their related reactions.
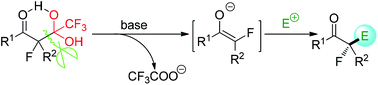
Org. Biomol. Chem., 2019,17, 762-775
https://doi.org/10.1039/C8OB02843E
Radical fluoroalkylation reactions of (hetero)arenes and sulfides under red light photocatalysis
The use of Zn-phthalocyanine in a red-light-driven process for the perfluoroalkylation reactions of (hetero)arenes and sufides represents a friendlier environmental alternative.

Org. Biomol. Chem., 2019,17, 3741-3746
https://doi.org/10.1039/C9OB00486F
Electrochemical trifluoromethylation/semipinacol rearrangement sequences of alkenyl alcohols: synthesis of β-CF3-substituted ketones
Electrochemical oxidative radical trifluoromethylation/semipinacol rearrangement sequences of alkenyl alcohols were developed in this study.
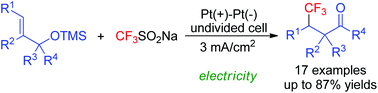
Org. Biomol. Chem., 2019,17, 3319-3323
https://doi.org/10.1039/C9OB00373H
Combined experimental and theoretical study of long-range H–F interactions in α-fluoro amides
A combined experimental and computational approach provided insight into the nature and conformational dependence of long-range 4JHF couplings in α-fluoro amides.

Chem. Commun., 2019,55, 2253-2256
https://doi.org/10.1039/C8CC09987A
Exploring physicochemical space via a bioisostere of the trifluoromethyl and ethyl groups (BITE): attenuating lipophilicity in fluorinated analogues of Gilenya® for multiple sclerosis
The BITE bioisostere of Et and CF3 allows lipophilicity to be tempered in analogues of the multiple sclerosis drug Gilenya®.
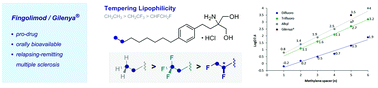
Chem. Commun., 2018,54, 12002-12005
https://doi.org/10.1039/C8CC05643A
Neutral and cationic tungsten(VI) fluoride complexes with tertiary phosphine and arsine coordination
The dodecahedral [WF4{o-C6H4(EMe2)2}2]2+ dications (E = P, As) present the highest oxidation state metal fluoride complexes with soft donor ligands.
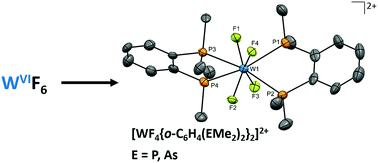
Chem. Commun., 2018,54, 11681-11684
https://doi.org/10.1039/C8CC05598J
A degradable fluorinated surfactant for emulsion polymerization of vinylidene fluoride
An original degradable fluorinated surfactant, 3-hydroxy-2-(trifluoromethyl)propanoic acid was applied for the emulsion polymerization of vinylidene difluoride (VDF) to yield a latex of PVDF, consisted of typically 100 nm particle diameters.
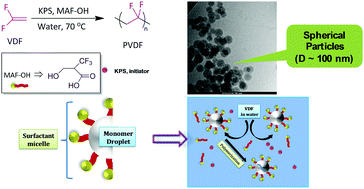
Chem. Commun., 2018,54, 11399-11402
https://doi.org/10.1039/C8CC05290E
Reactivity and properties of bis(chlorodifluoroacetyl) peroxide generated in situ from chlorodifluoroacetic anhydride for chlorodifluoromethylation reactions
A practical one-pot procedure using chlorodifluoroacetic anhydride as a user-friendly chlorodifluoromethyl-source was developed.

Chem. Commun., 2018,54, 11276-11279
https://doi.org/10.1039/C8CC05905E
A halogen bond-donor amino acid for organocatalysis in water
An XB-donor amino acid compound F(F4I) effectively promotes homogeneous catalysis of condensation reactions in water.
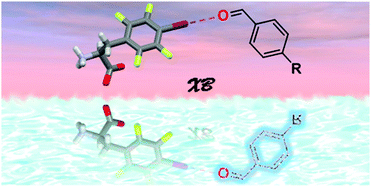
Chem. Commun., 2018,54, 10718-10721
https://doi.org/10.1039/C8CC06010J
C(sp2)–H Trifluoromethylation of enamides using TMSCF3: access to trifluoromethylated isoindolinones, isoquinolinones, 2-pyridinones and other heterocycles
A method for the direct C(sp2)–H trifluoromethylation of enamides, including biologically relevant isoindolinones, isoquinolinones and 2-pyridinones using TMSCF3 under oxidative conditions is presented.
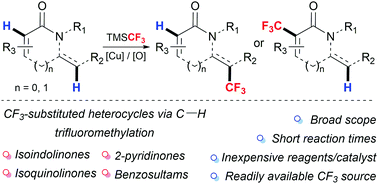
Chem. Commun., 2018,54, 10574-10577
https://doi.org/10.1039/C8CC04907F
Synthetic access to an elusive high-temperature perfluoroisopropenyl ether prepolymer for radical copolymerization
Generating a high yielding but strong fluorinated electrophile by destabilizing a low temperature perfluorocarbanion in non-polar solvent, while avoiding cascading side-reactions.
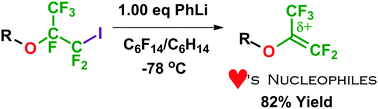
Chem. Commun., 2018,54, 10439-10442
https://doi.org/10.1039/C8CC05241G
Access towards enantiopure α,α-difluoromethyl alcohols by means of sulfoxides as traceless chiral auxiliaries
A new methodology has been developed to access highly enantioenriched α,α-difluoromethyl alcohols by means of sulfoxides as traceless and chiral auxiliaries.
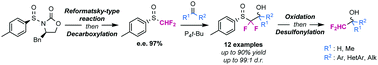
Chem. Commun., 2018,54, 10423-10426
https://doi.org/10.1039/C8CC05571H
Dramatic enhancement of spin–spin coupling and quenching of magnetic dimensionality in compressed silver difluoride
The high-pressure HP2 form of AgF2 features Ag2F73− units that are theoretically predicted to host extremely strong antiferromagnetic interactions, surpassing those seen in copper(II) oxides.
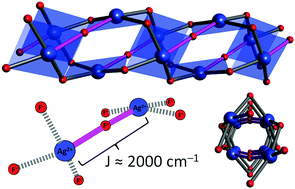
Chem. Commun., 2018,54, 10252-10255
https://doi.org/10.1039/C8CC05002C
Fast and reliable generation of [18F]triflyl fluoride, a gaseous [18F]fluoride source
Reactive [18F]fluoride was generated from gaseous [18F]triflyl fluoride and used for a wide range of radiofluorination reactions.
![Graphical abstract: Fast and reliable generation of [18F]triflyl fluoride, a gaseous [18F]fluoride source](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8CC03206H&imageInfo.ImageIdentifier.Year=2018)
Chem. Commun., 2018,54, 10179-10182
https://doi.org/10.1039/C8CC03206H
Controlled hydroxy-fluorination reaction of anatase to promote Mg2+ mobility in rechargeable magnesium batteries
Fluorination of anatase as an effective way to tune Mg2+ intercalation properties.
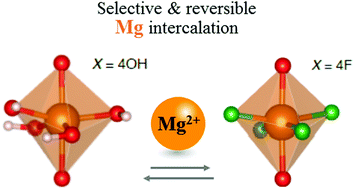
Chem. Commun., 2018,54, 10080-10083
https://doi.org/10.1039/C8CC04136A
Visible-light promoted fluoroalkylselenolation: toward the reactivity of unsaturated compounds
α-Fluoroalkylselenolated sulfones have been readily obtained via a visible-light promoted radical addition of fluoroalkylselenyl and tosyl groups onto unsaturated substrates.
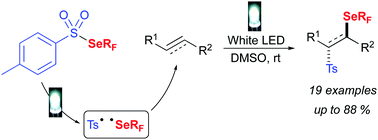
Chem. Commun., 2018,54, 9909-9912
https://doi.org/10.1039/C8CC05256E
Stereoselective aldol reactions using pseudo C2 symmetric 1-benzyl-4-(trifluoromethyl)piperidine-2,6-dione
Crossed aldol reactions of the CF3-containing pseudo C2 symmetric cyclic imide 3 were performed by way of the corresponding lithium and boron bisenolates to stereoselectively furnish the desired products 4.

Chem. Commun., 2018,54, 9913-9916
https://doi.org/10.1039/C8CC05458D
Photochemical activation of SF6 by N-heterocyclic carbenes to provide a deoxyfluorinating reagent
A photochemical activation of SF6 provides access to difluoroimidazolidine derivatives, which can serve as intermediates to shuttle fluorides to alcohols by deoxyfluorination.

Chem. Commun., 2018,54, 9753-9756
https://doi.org/10.1039/C8CC05494K
Design and synthesis of galactose-conjugated fluorinated and non-fluorinated proline oligomers: towards antifreeze molecules
Galactose-conjugated fluorinated and non-fluorinated proline oligomers were synthesized and evaluated as antifreeze molecules.
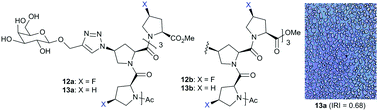
Chem. Commun., 2018,54, 9749-9752
https://doi.org/10.1039/C8CC05588B
Synthesis of new fluorinated proline analogues from polyfluoroalkyl β-ketoacetals and ethyl isocyanoacetate
New straightforward synthetic approach to hitherto unknown cis-/trans-CF3-prolines and other 3-polyfluoroalkyl proline analogues.

Chem. Commun., 2018,54, 9683-9686
https://doi.org/10.1039/C8CC05912H
Diels–Alder reactions of “in situ” generated perfluorinated thioketones
A simple and general procedure for the preparation of Diels–Alder adducts of perfluorinated thioketones and various dienes is reported.

Chem. Commun., 2018,54, 9298-9300
https://doi.org/10.1039/C8CC05075A
Tuning the Lewis acidity of difluorido gold(III) complexes: the synthesis of [AuClF2(SIMes)] and [AuF2(OTeF5)(SIMes)]
A route to [AuIIIClF2(SIMes)] and [AuIIIF2(OTeF5)(SIMes)] including structural characterization to tune the ligand arrangement and Lewis acidity of gold(III) centres was developed.
![Graphical abstract: Tuning the Lewis acidity of difluorido gold(iii) complexes: the synthesis of [AuClF2(SIMes)] and [AuF2(OTeF5)(SIMes)]](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8CC05233F&imageInfo.ImageIdentifier.Year=2018)
Chem. Commun., 2018,54, 9301-9304
https://doi.org/10.1039/C8CC05233F
The perfluorinated alcohols c-C6F11OH, c-C6F10-1,1-(OH)2 and c-C6F10-1-(CF3)OH
The thermally unstable α-fluoroalcohol undecafluorocyclohexanol (c-C6F11OH) was prepared by addition of hydrogen fluoride to the corresponding ketone.
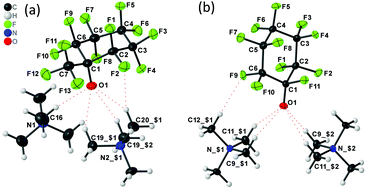
Chem. Commun., 2018,54, 9294-9297
https://doi.org/10.1039/C8CC05148H
Oximinotrifluoromethylation of unactivated alkenes under ambient conditions
An oximinotrifluoromethylation of unactivated alkenes was developed via trifluoromethyl radical-induced intramolecular remote oximino migration under mild reaction conditions.
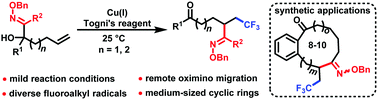
Chem. Commun., 2018,54, 8885-8888
https://doi.org/10.1039/C8CC05186K
Highly C-selective difluoromethylation of β-ketoesters by using TMSCF2Br/lithium hydroxide/N,N,N-trimethylhexadecan-1-ammonium bromide
The first highly C-selective difluoromethylation of β-ketoesters was achieved by combining TMSCF2Br/LiOH/(CH3(CH2)15(CH3)3NBr).
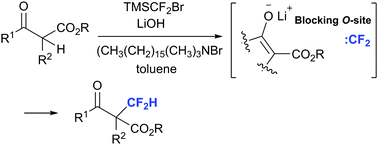
Chem. Commun., 2018,54, 8881-8884
https://doi.org/10.1039/C8CC05135F
The CF3-DAST-induced deacylative trifluoromethylthiolation of cyclic 1,3-diketones/lactams/lactones and its extension to deacylative pentafluorophenylthiolation
CF3-DAST-induced deacylative trifluoromethylthiolation of cyclic 1,3-diketones lactams, and lactones that provides cyclic trifluoromethylthioketones, lactams and lactones is reported.
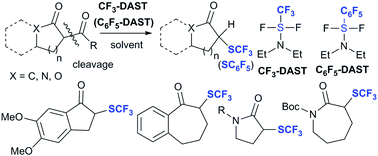
Chem. Commun., 2018,54, 8761-8764
https://doi.org/10.1039/C8CC05409F
Copper-mediated intramolecular aminofluorination of 1,3-dienes by using nucleophilic fluorine reagents
A copper-mediated intramolecular aminofluorination of 1,3-dienes is disclosed, in which both AgF and Et3N·3HF can be used as the fluorine source.
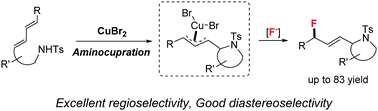
Chem. Commun., 2018,54, 8709-8712
https://doi.org/10.1039/C8CC04909B
Fluorinated cyclopropanes: synthesis and chemistry of the aryl α,β,β-trifluorocyclopropane motif
A general route to aryl α,β,β-trifluorocyclopropanes is reported and aryl oxidation gave the corresponding α,β,β-trifluorocyclopropane carboxylic acid.
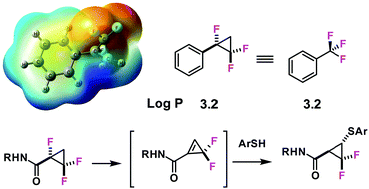
Chem. Commun., 2018,54, 8415-8418
https://doi.org/10.1039/C8CC04964E
Stille cross-coupling of secondary and tertiary α-(trifluoromethyl)-benzyl chlorides with allylstannanes
A Stille cross-coupling reaction was developed for the first time by secondary (trifluoromethyl)benzyl chlorides and allylstannanes.
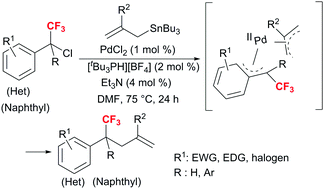
Chem. Commun., 2018,54, 7171-7174
https://doi.org/10.1039/C8CC03541E
Asymmetric synthesis of α-trifluoromethoxy ketones with a tetrasubstituted α-stereogenic centre via the palladium-catalyzed decarboxylative allylic alkylation of allyl enol carbonates
The Pd-catalyzed asymmetric decarboxylative allylic alkylation of trifluoromethoxy allyl enol carbonates is disclosed.

Chem. Commun., 2018,54, 5522-5525
https://doi.org/10.1039/C8CC03131B
Minimising conformational bias in fluoroprolines through vicinal difluorination
Combining the 4S-fluoro and 3R-fluoromotifs, which each instill an opposing conformational bias, leads to a fluorinated proline with a similar conformational profile to proline.
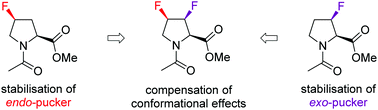
Chem. Commun., 2018,54, 5118-5121
https://doi.org/10.1039/C8CC01493K
Stereodivergent trifluoromethylation of N-sulfinylimines by fluoroform with either organic-superbase or organometallic-base
The first stereodivergent trifluoromethylation of N-sulfinylimines by fluoroform is reported.
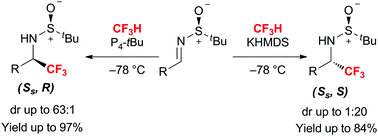
Chem. Commun., 2018,54, 4294-4297
https://doi.org/10.1039/C8CC01526K
A rhodium-catalyzed transannulation of N-(per)fluoroalkyl-1,2,3-triazoles under microwave conditions – a general route to N-(per)fluoroalkyl-substituted five-membered heterocycles
An efficient rhodium-catalyzed microwave-assisted transannulation of N-(per)fluoroalkyl-1,2,3-triazoles to previously inaccessible N-(per)fluoroalkyl five-membered heterocycles was developed.
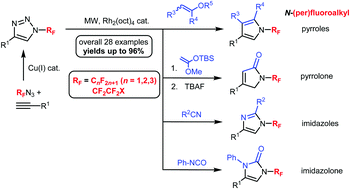
Chem. Commun., 2018,54, 3258-3261
https://doi.org/10.1039/C8CC01446A
Photoredox generation of the trifluoromethyl radical from borate complexes via single electron reduction
A trifluoromethyl radical is generated by single electron reduction of CF3-substituted borate complexes.
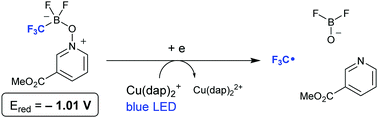
Chem. Commun., 2018,54, 2236-2239
https://doi.org/10.1039/C8CC00245B
Synthesis of (difluoromethyl)naphthalenes using the ring construction strategy: C–C bond formation on the central carbon of 1,1-difluoroallenes via Pd-catalyzed insertion
1,1-Difluoroallenes underwent β-selective C–C bond formation (insertion) via π-allylpalladium(II) to facilitate aromatic ring construction, leading to biologically promising (difluoromethyl)naphthalenes.

Org. Biomol. Chem., 2019,17, 5047-5054
https://doi.org/10.1039/C9OB00540D
Fluoroalkenylation of boronic acids via an oxidative Heck reaction
A fluoroalkenylation of boronic acids with fluoroalkyl alkenes has been developed.

Org. Biomol. Chem., 2019,17, 4317-4325
https://doi.org/10.1039/C9OB00332K
Visible-light-induced radical trifluoromethylthiolation of N-(o-cyanobiaryl)acrylamides
Visible-light-mediated trifluoromethylthiolation of N-(o-cyanobiaryl)acrylamides with N-trifluoromethylthiosaccharin as the source of SCF3 radicals provided an efficient route to SCF3-containing ring-fused phenanthridine derivatives.
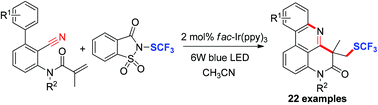
Org. Biomol. Chem., 2019,17, 3374-3380
https://doi.org/10.1039/C9OB00342H
Lewis acid promoted fluorine-alkoxy group exchange reactions for the synthesis of 5-alkoxy-4,4-difluoroisoxazoline systems
Lewis acid promoted substitution of fluorine yields novel 5-alkoxylated 4,4-difluoroisoxazolines via SN1 type processes.

Org. Biomol. Chem., 2019,17, 2818-2823
https://doi.org/10.1039/C9OB00097F
Tetrafluoropyridyl (TFP): a general phenol protecting group readily cleaved under mild conditions
Herein we introduce tetrafluoropyridyl (TFP) as a new general protecting group for phenols. The TFP protecting group is readily cleaved under mild conditions.
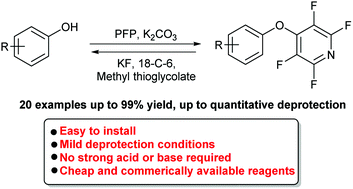
Org. Biomol. Chem., 2019,17, 2110-2115
https://doi.org/10.1039/C8OB02899K
Tuning the properties of a cyclic RGD-containing tetrapeptide through backbone fluorination
Fluorination alters a cyclic peptide's synthetic efficiency, its molecular conformation, and its biological activity.

Org. Biomol. Chem., 2019,17, 664-674
https://doi.org/10.1039/C8OB02679C
Synthesis of 4-trifluoromethyl 2-pyrones and pyridones through the Brønsted base-catalyzed Pechmann-type reaction with cyclic 1,3-diones
An efficient Brønsted base-catalyzed Pechmann-type reaction of cyclic 1,3-diones with ethyl 4,4,4-trifluoroacetoacetate to afford 4-trifluoromethyl 2-pyrones and pyridones was developed.
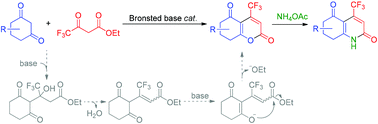
Org. Biomol. Chem., 2018,16, 9440-9445
https://doi.org/10.1039/C8OB02701C
Activation of the hypervalent fluoroiodane reagent by hydrogen bonding to hexafluoroisopropanol
Hexafluoroisopropan-2-ol is an excellent solvent for promoting fluorinations with the stable hypervalent fluoroiodane reagent without any transition metals or TREAT-HF activators.

Org. Biomol. Chem., 2018,16, 7170-7173
https://doi.org/10.1039/C8OB02236D
Synthesis of 2,2-difluoro-homoallylic alcohols via ring-opening of gem-difluorocyclopropane and aerobic oxidation by photo-irradiation in the presence of an organic pigment
The aerobic oxidation took place after the visible light-mediated ring-opening reaction of gem-difluorocyclopropane in the presence of an organic dye and amine to furnish 2,2-difluoro-homoallylic alcohols in good yields.
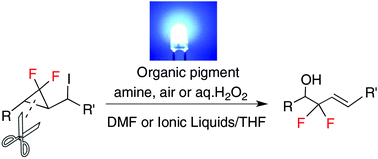
Org. Biomol. Chem., 2018,16, 6106-6114
https://doi.org/10.1039/C8OB01740A
About this collection
This collection, Guest Edited by ChemComm Chair Professor Veronique Gourvernor, has been created to compliment the 22nd International Symposium on Fluorine Chemistry. This collection is dedicated to all aspects of fluorine chemistry, celebrating the field’s current achievements and future perspectives. New articles will be added to this collection as they are published.