Themed collection New advances in catalytic C–C bond formation via late transition metals

Suzuki–Miyaura cross-couplings of secondary allylic boronic esters
The demonstration of a general method for cross-coupling of secondary allylic boronic esters was achieved for the first time under the same Pd-catalyzed conditions previously used for the coupling of benzylic substrates. Unlike allyl silanes, the regioselectivity of the arylation depends on the sterics and electronics of the allylic and vinylic substituents.
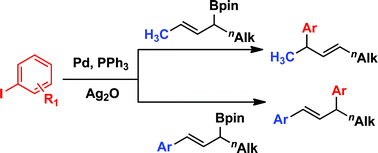
Chem. Commun., 2012,48, 1230-1232
https://doi.org/10.1039/C2CC16076E
Ligand -guided pathway selection in nickel-catalyzed couplings of enals and alkynes
Simple variation of phosphine structure allows selective access to alkylative couplings or reductive cycloadditions.
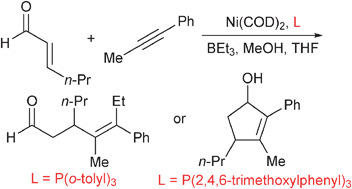
Chem. Commun., 2012,48, 1114-1116
https://doi.org/10.1039/C2CC17073F
Synthesis of chromans viaPd-catalyzed alkene carboetherification reactions
Palladium-catalyzed cross-coupling reactions between 2-(but-3-enyl)phenols and aryl or alkenyl bromides provide straightforward access to substituted chroman derivatives.

Chem. Commun., 2012,48, 609-611
https://doi.org/10.1039/C1CC15880E
Two-stage optimization of a supramolecular catalyst for catalytic asymmetric hydroboration
Systematic changes, first to the catalyst scaffold and then to the ligating groups, are successfully used to fine tune supramolecular catalyst structures.
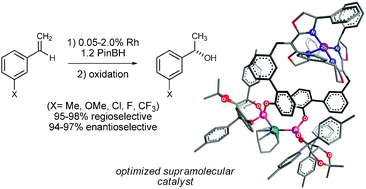
Chem. Commun., 2012,48, 263-265
https://doi.org/10.1039/C1CC16146F
Selective formation of angular tricyclic compounds by ruthenium-mediated ring-rearrangement metathesis
Angular tricyclic compounds with a variety of ring-sizes were produced by ring-rearrangement metathesis, sometimes with a high degree of selectivity.
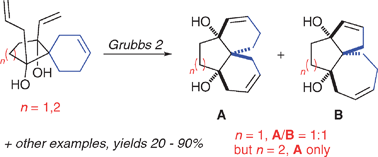
Chem. Commun., 2012,48, 233-235
https://doi.org/10.1039/C1CC15452D
Suzuki-Miyaura coupling of heteroaryl boronic acids and vinyl chlorides
A protocol based on a Pd(OAc)2/SPhos catalyst system for the Suzuki-Miyaura coupling of heteroaryl boronic acids and vinyl chlorides that minimizes protodeboronation is described.

Chem. Commun., 2012,48, 203-205
https://doi.org/10.1039/C1CC15990A
Regioselective C–H bond functionalizations of acridines using organozinc reagents
Regioselective C–H bond arylation and alkylation of acridines using organozinc reagents are described.
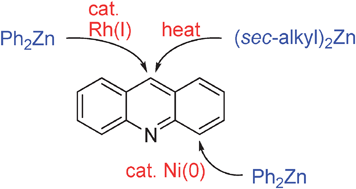
Chem. Commun., 2012,48, 308-310
https://doi.org/10.1039/C1CC16582H
Synthesis of 1,2,3-triazole -fused heterocycles viaPd-catalyzed cyclization of 5-iodotriazoles
A concise synthesis of fused 1,2,3-triazoles is described, featuring a palladium-catalyzed intramolecular Heck or direct arylation reaction.
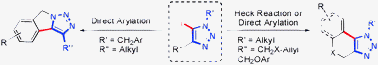
Chem. Commun., 2012,48, 55-57
https://doi.org/10.1039/C1CC16110E
Decarboxylative benzylation and arylation of nitriles
Decarboxylative coupling of benzylic and heterobenzylic esters results in either benzylation or arylation of nitrile-stabilized anions.
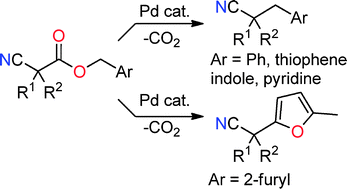
Chem. Commun., 2012,48, 142-144
https://doi.org/10.1039/C1CC16011G
Synthesis of diaryl ketonesvia a phosphine -free Fukuyama reaction
An efficient method to form unsymmetrical diaryl ketones via Fukuyama cross-coupling reactions with low loadings of Pd(dba)2 has been developed.
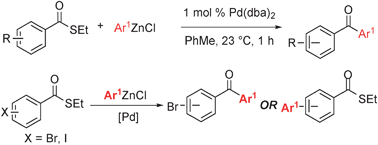
Chem. Commun., 2011,47, 12679-12681
https://doi.org/10.1039/C1CC16114H
Rhodium(III)-catalyzed oxidative carbonylation of benzamides with carbon monoxide
A mild Rh-catalyzed carbonylative coupling of benzamides to form phthalimides has been developed.
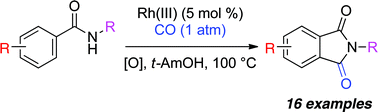
Chem. Commun., 2011,47, 12074-12076
https://doi.org/10.1039/C1CC15843K
Pyridine synthesis from oximes and alkynesviarhodium(III) catalysis: Cp* and Cpt provide complementary selectivity
The use of sterically different ligands in the synthesis of pyridines from oximes and alkynes allows for complementary selectivities to be achieved.
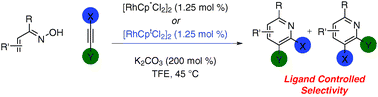
Chem. Commun., 2011,47, 11846-11848
https://doi.org/10.1039/C1CC15248C
Regiocontrolled aerobic oxidative coupling of indoles and benzene using Pd catalysts with 4,5-diazafluorene ligands
Diazafluorene-derived ligands enable regiocontrolled Pd-catalyzed oxidative coupling of indoles and benzene to proceed with O2 as the oxidant.
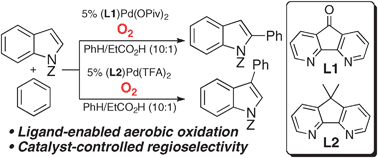
Chem. Commun., 2011,47, 10257-10259
https://doi.org/10.1039/C1CC13632A
Cobalt -catalyzed conjugate addition of silylacetylenes to α,β-unsaturated ketones
Catalytic addition of silylacetylenes to α,β-unsaturated ketones proceeded in the presence of a cobalt complex coordinated with a bisphosphine ligand to give high yields of β-alkynylketones.

Chem. Commun., 2011,47, 10142-10144
https://doi.org/10.1039/C1CC14098A
Catalytic enantioselective Grignard Nozaki–Hiyama methallylation from the alcohol oxidation level: chloride compensates for π-complex instability
Methallyl chloride serves as an efficient allyl donor in highly enantioselective Grignard Nozaki–Hiyama methallylations from the alcohol or aldehyde oxidation level via iridium catalyzed transfer hydrogenation. Under identical conditions, methallyl acetate does not react efficiently.
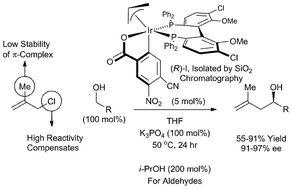
Chem. Commun., 2011,47, 10028-10030
https://doi.org/10.1039/C1CC14392A
Synthesis of epoxybenzo[d]isothiazole 1,1-dioxides via a reductive-Heck, metathesis-sequestration protocol
An atom-economical, purification free method for the synthesis of tricyclic sultams has been developed utilizing a facilitated ring-opening metathesis polymerization (ROMP) protocol.
![Graphical abstract: Synthesis of epoxybenzo[d]isothiazole 1,1-dioxides via a reductive-Heck, metathesis-sequestration protocol](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C1CC12503F&imageInfo.ImageIdentifier.Year=2011)
Chem. Commun., 2011,47, 9528-9530
https://doi.org/10.1039/C1CC12503F
Gold-catalysed alkenyl - and arylsilylation reactions forming 1-silaindenes
In the presence of gold(I)–phosphine catalysts, alkenyl- and arylsilanes undergo intramolecular cyclisation reactions onto appendant alkyne moieties to afford 1-silaindene derivatives. The reaction pathways vary depending on the substituent on silicon.
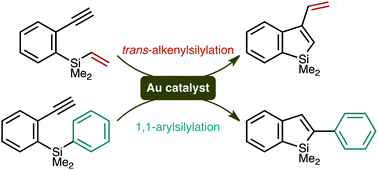
Chem. Commun., 2011,47, 8697-8699
https://doi.org/10.1039/C1CC12457A
Control of selectivity in the generation and reactions of oxonium ylides
trans-6-Aryl-4-keto-2-pyranyl-diazoacetoacetates undergo the [1,2]-Stevens rearrangement in a controllable conformation-dependent process.
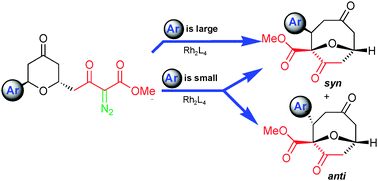
Chem. Commun., 2011,47, 7623-7625
https://doi.org/10.1039/C1CC12443A
Efficient palladium -catalyzed N-arylation of a sulfoximine with aryl chlorides
Aryl chlorides are useful substrates for the palladium-catalyzed N-arylation of a sulfoximine when RuPhos is used as a ligand.

Chem. Commun., 2011,47, 7665-7667
https://doi.org/10.1039/C1CC12444G
Au-catalyzed synthesis of 2-alkylindoles from N-arylhydroxylamines and terminal alkynes
2-Alkylindoles are prepared regiospecifically in a gold-catalyzed bimolecular process under exceedingly mild reaction conditions.

Chem. Commun., 2011,47, 7815-7817
https://doi.org/10.1039/C1CC12212F
Catalytic asymmetric hydroboration of β,γ-unsaturated Weinreb amides : striking influence of the borane
Subtle differences in borane structure strongly influence the efficiency of catalytic asymmetric hydroboration with β,γ-unsaturated Weinreb amide substrates.
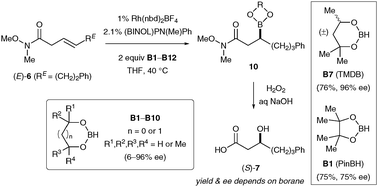
Chem. Commun., 2011,47, 7812-7814
https://doi.org/10.1039/C1CC11746G
A highly active and selective palladium pincer catalyst for the formation of α-aryl ketonesvia cross-coupling
The air and moisture stable Pd complex [t-BuPhebox-Me2]PdBr is capable of efficiently promoting α-monoarylation of ketones with numerous aryl bromides in 1 h at 70 °C with 82–99% yields.
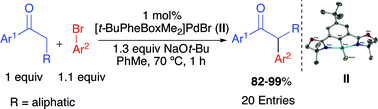
Chem. Commun., 2011,47, 7218-7220
https://doi.org/10.1039/C1CC12071A
Solvent controlled mechanistic dichotomy in a Au(III)-catalyzed, heterocyclization triggered, Nazarov reaction
Tandem Au(III)-catalyzed heterocyclization/Nazarov cyclizations leading to substituted carbocycle fused furans are described. An interesting dichotomy of reaction pathways as a function of solvent, confirmed by the isolation and trapping of reaction intermediates, provided a basis for computational studies that supported the experimental findings.
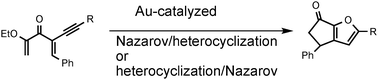
Chem. Commun., 2011,47, 6707-6709
https://doi.org/10.1039/C1CC10920K
Pd(II)-catalyzed decarboxylative cross-coupling of oxamic acids with potassium phenyltrifluoroborates under mild conditions
A novel Pd-catalyzed decarboxylative cross-coupling of oxamic acids with potassium phenyltrifluoroborates has been realized under mild reaction conditions.

Chem. Commun., 2011,47, 6587-6589
https://doi.org/10.1039/C1CC11635E
Pd(0)/Au(I) redox incompatibilities as revealed by Pd-catalyzed homo-coupling of arylgold(I)-complexes
A Pd(0)/Au(I) redox-driven palladium catalyzed homo-coupling of gold–aryl complexes illustrates potential redox incompatibilities of both metals for the development of Pd/Au dual catalysis.
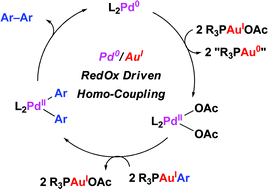
Chem. Commun., 2011,47, 5172-5174
https://doi.org/10.1039/C1CC11055A
Increasing synthetic efficiency via direct C–H functionalization: formal synthesis of an inhibitor of botulinum neurotoxin
A new synthesis of an inhibitor of botulinum neurotoxin is reported which involves the formal activation of three C–H bonds.
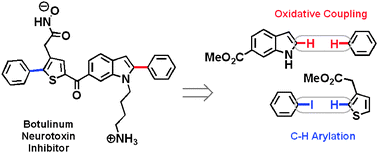
Chem. Commun., 2011,47, 4679-4681
https://doi.org/10.1039/C1CC10755K
Highly efficient desymmetrisation of a tricarbonylchromium 1,4-dibromonaphthalene complex by asymmetric Suzuki–Miyaura coupling
An efficient protocol is described for the desymmetrisation of a prochiral complex by catalytic desymmetrisation via an asymmetric Suzuki–Miyaura coupling reaction, using a bulky chiral palladium catalyst.
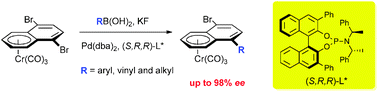
Chem. Commun., 2011,47, 3739-3741
https://doi.org/10.1039/C1CC10347D
Nickel-catalyzed intermolecular codimerization of acrylates and alkynes
The codimerization of acrylates and alkynes to produce 1,3-dienes is demonstrated using a nickel catalyst in association with 2-aminopyridine.
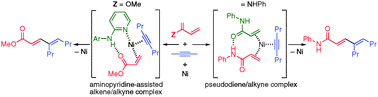
Chem. Commun., 2011,47, 2658-2660
https://doi.org/10.1039/C0CC04061D
Room-temperature nickel-catalysed cross-couplings of aryl chlorides with arylzincs
P,N,O-chelate nickel complexes efficiently catalyse the cross-coupling reaction of arylzinc reagents with aryl chlorides at room temperature with low catalyst loading.
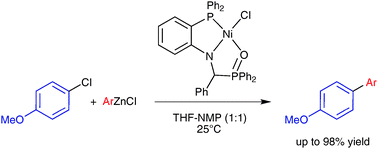
Chem. Commun., 2011,47, 1598-1600
https://doi.org/10.1039/C0CC03064C
Gold(I)-catalysed synthesis of conjugated trienes
Gold(I)-catalysed reaction between cyclopropenes and furans produces functionalised conjugated trienes.
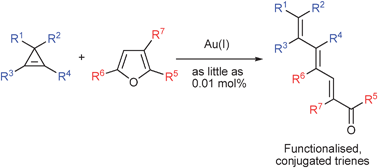
Chem. Commun., 2011,47, 1333-1335
https://doi.org/10.1039/C0CC04217J
Preparation of benzolactams by Pd(II)-catalyzed carbonylation of N-unprotected arylethylamines
An unprecedented NH2-directed Pd(II)-catalytic carbonylation of quaternary N-unprotected arylethylamines to yield selectively 6-membered benzolactams has been developed.
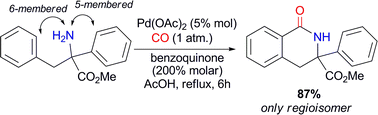
Chem. Commun., 2011,47, 1054-1056
https://doi.org/10.1039/C0CC03478A
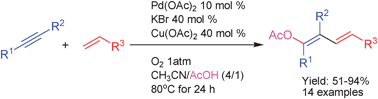
Acetoxypalladation of unactivated alkynes and capture with alkenes to give 1-acetoxy-1,3-dienes taking dioxygen as terminal oxidant
A general protocol for the synthesis of 1-acetoxy-1,3-dienes from alkynes and alkenes by an acetoxypalladation/Heck cross-coupling/β-H elimination tandem process is described.
Chem. Commun., 2011,47, 1003-1005
https://doi.org/10.1039/C0CC03723K
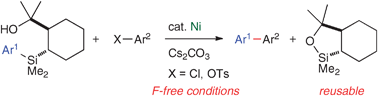
Nickel-catalysed cross-coupling reaction of aryl(trialkyl)silanes with aryl chlorides and tosylates
Nickel-catalysed cross-coupling reactions of aryl(trialkyl)silanes with aryl chlorides and tosylates are described.
Chem. Commun., 2011,47, 307-309
https://doi.org/10.1039/C0CC02173C
About this collection
The invited, peer-reviewed articles in this web themed issue highlight cutting-edge contributions by international leaders and celebrate current achievements and future perspectives in this exciting field of research.
The guest editor of this issue is Michael Krische (University of Texas at Austin).
Articles in this web themed issue will be added to the list below as soon as possible, after they are published. Please return to this page frequently to see this collection grow.
For information about submitting to this issue, please see the ChemComm blog .