Themed collection Selective Catalysis for Organic Synthesis

Chiral Pd aqua complex-catalyzed asymmetric C–C bond-forming reactions: a Brønsted acid–base cooperative system
Chiral cationic Pd aqua complexes can function as acid–base catalysts to give chiral Pd enolates with the concomitant formation of a protic acid. Cooperative action of the chiral Pd enolates with the protic acid to activate electrophiles is important to promote various C–C bond-forming reactions smoothly in a highly enantioselective manner.
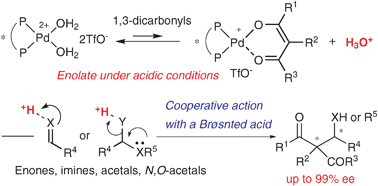
Chem. Commun., 2009, 5787-5798
https://doi.org/10.1039/B911015A
Mass spectrometric screening of chiral catalysts and catalyst mixtures
The use of quasi-enantiomeric substrates and ESI-MS makes it possible to determine the intrinsic enantioselectivity of chiral catalysts by monitoring catalytic intermediates, using a fast, operationally simple protocol.
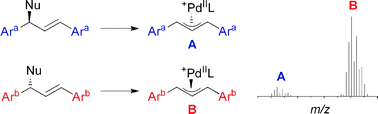
Chem. Commun., 2009, 1607-1618
https://doi.org/10.1039/B822382C
Transition metal-catalyzed addition reactions of arylboronic acids with alkyl 2-formylbenzoates: efficient access to chiral 3-substituted phthalides
Transition metal-catalyzed addition of arylboronic acids to 2-formylbenzoates afforded 3-substituted phthalides. By using SPINOL-based phosphites as ligands, a Rh(I)-catalyzed asymmetric version of such an addition reaction has been realized and up to 83% ee was achieved.
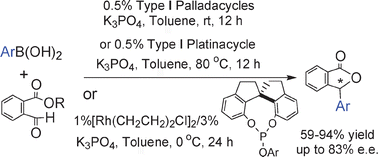
Chem. Commun., 2010,46, 3010-3012
https://doi.org/10.1039/C001104E
Total synthesis of (+)-scyphostatin featuring an enantioselective and highly efficient route to the side-chain via Zr -catalyzed asymmetric carboalumination of alkenes (ZACA )
An enantioselective ZACA route to the side-chain 3 permits an efficient and convergent synthesis of (+)-scyphostatin (1).
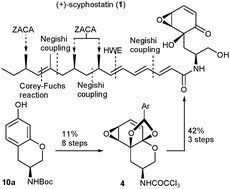
Chem. Commun., 2010,46, 2200-2202
https://doi.org/10.1039/B920261G
Diastereoselective and enantioselective Mukaiyama aldol reactions of α-ketoesters using hydrogen bond catalysis
Hydrogen bond catalyzed Mukaiyama aldol reactions of α-ketoesters proceed in high diastereo- and enantioselectivities, giving products possessing two chiral centers, of which one is a tertiary alcohol.
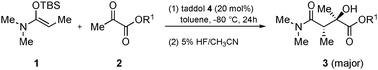
Chem. Commun., 2010,46, 904-906
https://doi.org/10.1039/B919929B
Use of achiral additives to increase the stereoselectivity in Rh(II) -catalyzed cyclopropanations
We describe our studies on the effect of various Lewis bases and Brønsted acids as achiral additives on the stereoselectivity of some Rh(II)-catalyzed cyclopropanations.

Chem. Commun., 2010,46, 910-912
https://doi.org/10.1039/B920587J
Rhodium -catalyzed dehydrogenative borylation of cyclic alkenes
A rhodium-catalyzed dehydrogenative borylation of cyclic alkenes is described. This reaction provides direct access to cyclic 1-alkenylboronic acid pinacol esters, useful intermediates in organic synthesis. Suzuki–Miyaura cross-coupling applications are also presented.
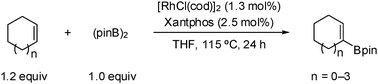
Chem. Commun., 2010,46, 907-909
https://doi.org/10.1039/B921387B
Gold(I) -catalysed cycloisomerisation of 1,6-enynes into functionalised allenes
1,6-Enynes can be cycloisomerised into allenes under gold catalysis. This transformation involves a 5-exo dig cyclisation followed by 1,5-hydride shift.
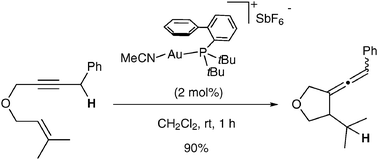
Chem. Commun., 2010,46, 865-867
https://doi.org/10.1039/B919240A
One pot ‘click’ reactions: tandem enantioselective biocatalytic epoxide ring opening and [3+2] azide alkyne cycloaddition
Halohydrin dehalogenase can perform enantioselective azidolysis of aromatic epoxides to 1,2-azido alcohols which are ligated to alkynes producing chiral β-hydroxy-triazoles in a one-pot procedure with excellent enantiomeric excess.
![Graphical abstract: One pot ‘click’ reactions: tandem enantioselective biocatalytic epoxide ring opening and [3+2] azide alkyne cycloaddition](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=B919434G&imageInfo.ImageIdentifier.Year=2010)
Chem. Commun., 2010,46, 898-900
https://doi.org/10.1039/B919434G
Cross-couplings between benzylic and aryl halides “on water ”: synthesis of diarylmethanes
Diarylmethanes are made “on water” simply upon mixing, in water: reactants, catalyst, and a touch of TMEDA, at room temperature. Voila.

Chem. Commun., 2010,46, 562-564
https://doi.org/10.1039/B922280D
Synthesis of 3-hydroxyoxindoles by Pd -catalysed intramolecular nucleophilic addition of aryl halides to α-ketoamides
Pd/PtBu3-catalysed intramolecular nucleophilic addition of aryl halides to α-ketoamides in the presence of nBuOH and base has been realized with high yields, providing a new, direct, and efficient synthetic strategy to obtain 3-hydroxyoxindoles.

Chem. Commun., 2010,46, 130-132
https://doi.org/10.1039/B917958E
Pd -catalyzed cascade carbopalladation-annulation reaction of 3-(2-iodobenzyl)-indoles into fused 6/5/7/6- and 6/5/5/6- heterocyclic systems
Polycyclic indole structures, possessing fused seven-membered rings were efficiently synthesized via the Pd-catalyzed intramolecular carbopalladation-annulation of 3-(2-iodobenzyl)-indoles with alkynes.
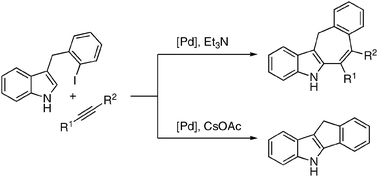
Chem. Commun., 2010,46, 150-152
https://doi.org/10.1039/B919991H
Highly enantioselective hydrogenation of α-aryl-β-substituted acrylic acids catalyzed by Ir-SpinPHOX
The enantioselective hydrogenation of a series of challenging substrates, α-aryl-β-substituted acrylic acids, was realized with high efficiency and enantioselectivity (up to 96%) under the catalysis of Ir(I) complex of Spiro-based P,N ligand, SpinPHOX.

Chem. Commun., 2010,46, 156-158
https://doi.org/10.1039/B919902K
Subtleties in asymmetric catalyst structure: the resolution of a 6-phospha-2,4,8-trioxa -adamantane and its applications in asymmetric hydrogenation catalysis
Resolution of a C1-symmetric phospha-adamantane which has the diamondoid structure shown has been achieved and, surprisingly, its Rh complex is a selective asymmetric hydrogenation catalyst.
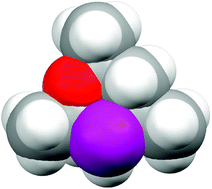
Chem. Commun., 2010,46, 100-102
https://doi.org/10.1039/B916359J
Silver(I)-mediated highly enantioselective synthesis of axially chiral allenes under thermal and microwave -assisted conditions
Silver(I) salts mediated stereospecific transformation of optically active propargylamines to axially chiral allenes with excellent enantioselectivities (17 examples with 96–99% ee; one substrate with 91% ee) without subsequent racemization.
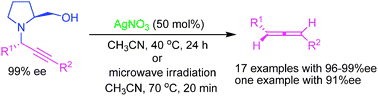
Chem. Commun., 2010,46, 213-215
https://doi.org/10.1039/B914516H
Chiral bifunctional phase transfer catalysts for asymmetric fluorination of β-keto esters
Chiral bifunctional phase transfer catalysts introducing bis(diarylhydroxymethyl) substituents at 3,3′-positions of the chiral binaphthyl core were successfully applied to asymmetric fluorination of cyclic β-keto esters with high enantioselectivities.

Chem. Commun., 2010,46, 321-323
https://doi.org/10.1039/B920099A
Stereoselective synthesis of trans-β-lactams by palladium -catalysed carbonylation of vinyl aziridines
The palladium-catalysed carbonylation of vinyl aziridines can give either trans- or cis-β-lactams preferentially or even the δ-lactam simply by adjusting the reaction parameters ([Pd], [CO], temperature).

Chem. Commun., 2010,46, 267-269
https://doi.org/10.1039/B920564K
Palladium -catalyzed [3C + 2C + 2C ] cycloaddition of enynylidenecyclopropanes: efficient construction of fused 5-7-5 tricyclic systems
A Pd-catalyzed intramolecular [3C + 2C + 2C] cycloaddition between alkylidenecyclopropanes, alkynes and alkenes is reported. The method provides synthetically relevant 5-7-5 tricyclic structures, with good chemoselectivity and complete diastereoselectivity.
![Graphical abstract: Palladium-catalyzed [3C + 2C + 2C] cycloaddition of enynylidenecyclopropanes: efficient construction of fused 5-7-5 tricyclic systems](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=B919258A&imageInfo.ImageIdentifier.Year=2010)
Chem. Commun., 2010,46, 270-272
https://doi.org/10.1039/B919258A
Organocatalytic asymmetric Povarov reactions with 2- and 3-vinylindoles
The organocatalytic asymmetric Povarov reaction of N-arylimines with 2- and 3-vinylindoles has been developed. The peculiar reactivity of vinylindoles allowed the disclosure of versatile reaction manifolds for the preparation of highly enantioenriched indole derivatives.
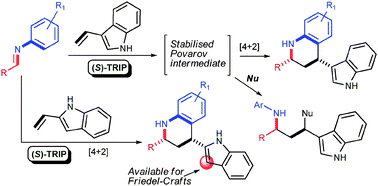
Chem. Commun., 2010,46, 327-329
https://doi.org/10.1039/B921113F
Stereoselective gold-catalyzed cycloaddition of functionalized ketoenynes: synthesis of (+)-orientalol F
The synthesis of (+)-orientalol F has been completed by a stereoselective gold-catalyzed [2 + 2 + 2] cycloaddition of a ketoenyne, in which the propargylic stereocenter controls the formation of three additional stereocenters.
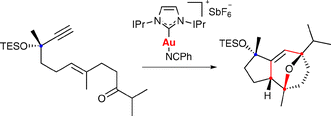
Chem. Commun., 2009, 7327-7329
https://doi.org/10.1039/B920119J
Highly enantioselective hetero-Diels–Alder reactions between Rawal’s diene and aldehydes catalyzed by chiral dirhodium(II) carboxamidates
Rh2(S-BPTPI)4 is an exceptionally effective catalyst for enantioselective hetero-Diels–Alder reactions between Rawal’s diene and a diverse range of aldehydes to give, after treatment with acetyl chloride, dihydropyranone derivatives in good yields and with excellent enantioselectivities.
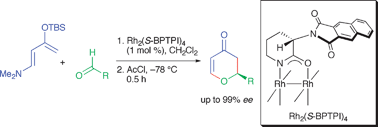
Chem. Commun., 2010, 7294-7296
https://doi.org/10.1039/B919535A
Iron-catalyzed addition of Grignard reagents to activated vinyl cyclopropanes
The iron-catalyzed addition of Grignard reagents to activated vinyl cyclopropanes proceeds in a highly regioselective fashion for branched primary, secondary and tertiary alkyl Grignard reagents. The observed selectivity likely results from a direct addition mechanism as opposed to single electron transfer or an iron-allyl based process.
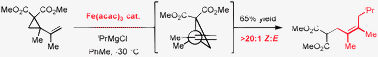
Chem. Commun., 2009, 7116-7118
https://doi.org/10.1039/B918818E
γ-Agostic interactions stabilize the propagating species in the vinyl addition polymerization of norbornene
Cationic [(tmeda)Pd(OEt2)(Me)][B(ArF)4] was synthesized and allowed for the first time the nature of the propagating species in the vinyl addition polymerization of norbornene to be discerned. In this system γ-agostic interactions stabilize the propagating species and permit stepwise monomer insertions to be observed by 1H NMR spectroscopy.
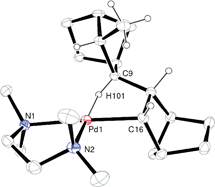
Chem. Commun., 2009, 6361-6363
https://doi.org/10.1039/B916457J
Copper-catalyzed arene C–H bond cross-coupling
A highly regioselective, one-pot sequential iodination–copper-catalyzed cross-coupling of arene C–H bonds has been developed affording an efficient method for biaryl synthesis.
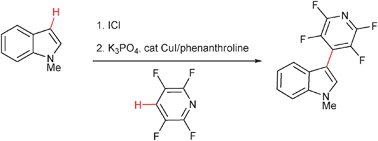
Chem. Commun., 2009, 6433-6435
https://doi.org/10.1039/B912890E
Regioselective synthesis of halohydrin esters from epoxides : reaction with acyl halides and rhodium-catalyzed three-component coupling reaction with alkyl halides and carbon monoxide
Regioselective synthesis of halohydrin esters was achieved by (1) the reaction of acyl halides with epoxides and (2) the rhodium-catalyzed three-component coupling reaction of alkyl halides, carbon monoxide, and epoxides.

Chem. Commun., 2009, 6970-6972
https://doi.org/10.1039/B912907C
Solid-phase synthesis of protected α-amino phosphonic acid oligomers
By establishing both a highly efficient phosphonamidate formation and a RuCp-catalyzed cleavage of an allyl linker, the solid-phase synthesis of Fmoc-(GlyP(OBn))6-OH/DIEA, a protected form of a new type of unnatural peptide α-amino phosphonic acid oligomer (APO), has been realized.
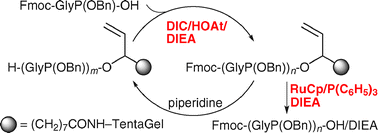
Chem. Commun., 2009, 6985-6987
https://doi.org/10.1039/B912231A
Palladium -catalyzed aryl halide carbonylation –intramolecular O-enolate acylation : efficient isocoumarin synthesis, including the synthesis of thunberginol A
Balloon pressure of CO is sufficient to efficiently convert a series of α-(o-haloaryl)-substituted ketones to the corresponding isocoumarins under the action of palladium catalysis.
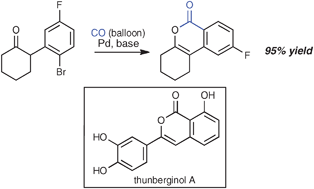
Chem. Commun., 2009, 6744-6746
https://doi.org/10.1039/B917839B
Getting the sterics just right: a five-coordinate iridium trisboryl complex that reacts with C–H bonds at room temperature
Synthesis of five-coordinate boryl complexes that react directly with arenes at ambient temperature provides the first glimpse of the fundamental step in Ir-catalyzed C–H borylation.
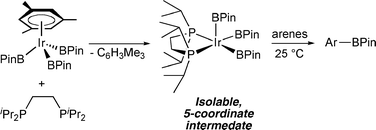
Chem. Commun., 2009, 5731-5733
https://doi.org/10.1039/B914736E
Palladium-catalysed arylative cyclisation of N-allylacetamides with aryl halides yielding benzyl -substituted oxazolines
Treatment of N-allylacetamide with aryl halide in the presence of sodium t-butoxide and a palladium catalyst leads to arylative cyclisation to provide the corresponding benzyl-substituted oxazoline in high yield.

Chem. Commun., 2009, 5754-5756
https://doi.org/10.1039/B912895F
β-Amidoaldehydes viaoxazoline hydroformylation
4-Substituted oxazolines, which are readily synthesized from naturally occurring α-amino acids, are converted efficiently and stereospecifically to β-amidoaldehydes in the presence of synthesis gas and catalytic dicobalt octacarbonyl.
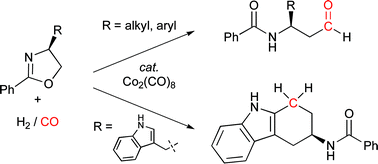
Chem. Commun., 2009, 5704-5706
https://doi.org/10.1039/B913698C
Metal-catalyzed rearrangement of enantiomerically pure alkylidenecyclopropane derivatives as a new access to cyclobutenes possessing quaternary stereocenters
The Pt(II)- and Pd(II)-catalyzed ring-expansion of alkylidenecyclopropane derivatives leads to the formation of cyclobutenes in good yields. The presence of an enantiomerically pure quaternary stereocenter on the alkylidenecyclopropane gives the corresponding cyclobutenes with the same enantiomeric ratio.
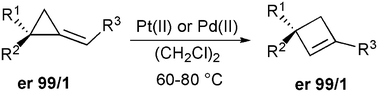
Chem. Commun., 2009, 5760-5762
https://doi.org/10.1039/B910465H
Asymmetric meso-aziridine ring-opening reactions using a chiral zirconium catalyst
We report asymmetric ring-opening reactions of meso-N-benzhydryl aziridines with anilines catalyzed by a Zr-BINOLate complex.
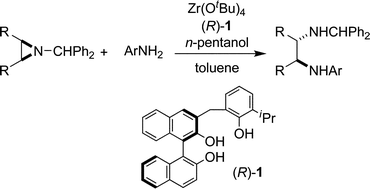
Chem. Commun., 2009, 5722-5724
https://doi.org/10.1039/B914271C
The concise synthesis of chiral tfb ligands and their application to the rhodium-catalyzed asymmetric arylation of aldehydes
New C2-symmetric tetrafluorobenzobarrelene ligands were prepared and applied successfully to the rhodium-catalyzed asymmetric addition of arylboronic acids to aromatic aldehydes giving chiral diarylmethanols in high yield with high enantioselectivity.
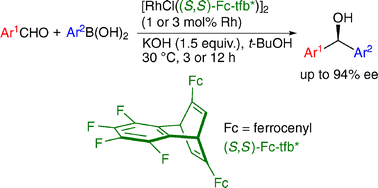
Chem. Commun., 2009, 5713-5715
https://doi.org/10.1039/B911118B
NeoPHOX—an easily accessible P,N-ligand for iridium-catalyzed asymmetric hydrogenation : preparation, scope and application in the synthesis of demethyl methoxycalamenene
Using a new class of chiral iridium hydrogenation catalysts, the antitumor natural product demethyl calamenene was synthesized in four steps in >20% overall yield and high enantiomeric purity.
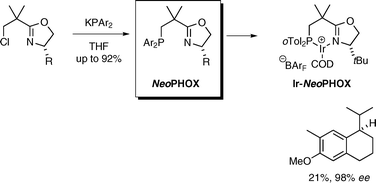
Chem. Commun., 2009, 6210-6212
https://doi.org/10.1039/B912680E
Acid-mediated formation of trifluoromethyl sulfonates from sulfonic acids and a hypervalent iodine trifluoromethylating agent
A new, straightforward synthesis of trifluoromethyl sulfonates, a class of virtually unexplored compounds: another surprising reaction of the hypervalent iodine reagent 1-trifluoromethyl-1,2-benziodoxol-3(1H)-one.
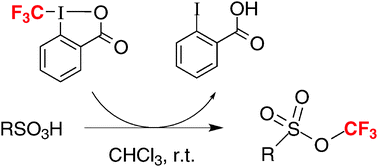
Chem. Commun., 2009, 5993-5995
https://doi.org/10.1039/B913962A
Iridium-catalyzed enantioselective hydrogenation of vinyl boronates
The first Ir-catalyzed asymmetric hydrogenations of vinyl boronates have been performed using low catalyst loadings and pressure (as low as 1 bar). Good selectivities (76–98% ee) were obtained for a range of substrates.
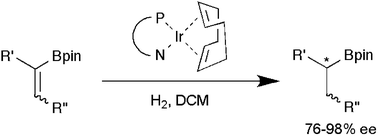
Chem. Commun., 2009, 5996-5998
https://doi.org/10.1039/B912590F
Asymmetric β-boration of α,β-unsaturated carbonyl compounds promoted by chiral rhodium–bisoxazolinylphenyl catalysts
Enantioselective β-boration of α,β-unsaturated carbonyl compounds with up to 97% ee with bis(pinacolato)diboron was attained with chiral rhodium–bisoxazolinylphenyl acetate complexes.
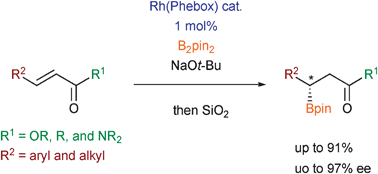
Chem. Commun., 2009, 5987-5989
https://doi.org/10.1039/B915759J
Direct functionalization of benzylic C–Hs with vinyl acetates via Fe-catalysis
Direct cross-coupling to construct sp3 C–sp3 C bonds via Fe-catalyzed benzylic C–H activation with 1-aryl vinyl acetate is described. The first Heck-type olefination of benzylic C–Hs was also observed with styrene as a reagent.
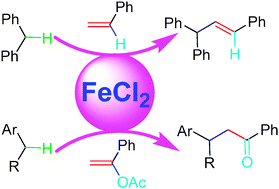
Chem. Commun., 2009, 6002-6004
https://doi.org/10.1039/B911031C
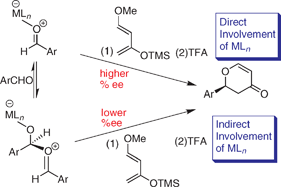
Barriers to enantiocontrol in Lewis acid catalyzed hetero-Diels–Alder reactions
Lewis bases inhibit the rate of conversion for cycloaddition and those, like aldehydes or nitriles, cause a decrease in enantiocontrol due to the Lewis acidity of the activated complex.
Chem. Commun., 2009, 5612-5614
https://doi.org/10.1039/B913019E
Platinum-catalysed aerobic 1,2-aminooxygenation of alkenes
Platinum(II) salts in combination with copper salts and molecular oxygen catalyse an unprecedented aerobic alkene oxidation that yields cyclic aminooxygenation products under sustainable conditions.

Chem. Commun., 2009, 5591-5593
https://doi.org/10.1039/B912139K
Pronounced effects of substituents on the iridium-catalyzed borylation of aryl C–H bonds
Iridium trisboryl complexes containing bisphosphine and bipyridine ligands and pinacolate and catecholate substituents are reported.

Chem. Commun., 2009, 5603-5605
https://doi.org/10.1039/B913949D
Total synthesis of (+)-virgatusin viaAlCl3 -catalyzed [3+2] cycloaddition
A stereocontrolled asymmetric synthesis of virgatusin was achieved in five steps from donor–acceptor cyclopropane 1 employing a Lewis acid-catalyzed cyclopropane–aldehyde addition reaction that constructs the tetrahydrofuran.
![Graphical abstract: Total synthesis of (+)-virgatusinviaAlCl3-catalyzed [3+2] cycloaddition](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=B911765B&imageInfo.ImageIdentifier.Year=2009)
Chem. Commun., 2009, 5135-5137
https://doi.org/10.1039/B911765B
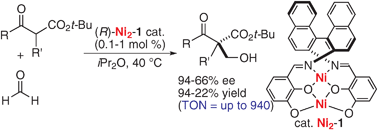
Direct catalytic asymmetric aldol reaction of β-keto esters with formaldehyde promoted by a dinuclear Ni2-Schiff base complex
A homodinuclear Ni2-Schiff base-catalyzed direct asymmetric hydroxymethylation of β-keto esters gave products in up to 94% ee with catalyst turnover number (TON) up to 940.
Chem. Commun., 2009, 5138-5140
https://doi.org/10.1039/B912380F
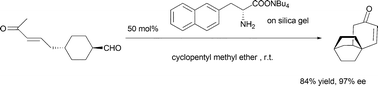
Amino acid salt catalyzed intramolecular Robinson annulation
The silica gel absorbed amino acid salt catalyzed asymmetric intramolecular Robinson annulation reaction has been developed; up to 97% ee was obtained with this readily recoverable organocatalyst.
Chem. Commun., 2009, 5412-5414
https://doi.org/10.1039/B912325C
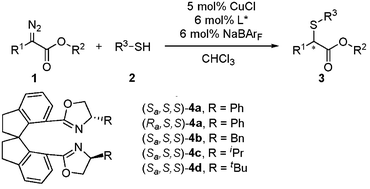
Copper-catalyzed enantioselective carbenoid insertion into S–H bonds
An asymmetric carbenoid insertion into S–H bonds catalyzed by copper–chiral spiro bisoxazoline complexes has been developed. A series of α-mercaptoesters were produced in high yields with moderate to good enantioselectivities (up to 85% ee). This result represents the best enantioselectivity in the catalytic asymmetric carbenoid S–H bond insertion reaction.
Chem. Commun., 2009, 5362-5364
https://doi.org/10.1039/B911670B
Efficient syntheses of α-pyridones and 3(2H)-isoquinolones through ruthenium-catalyzed cycloisomerization of 3-en-5-ynyl and o-alkynylphenyl nitrones
We report a new catalytic synthesis of α-pyridones and 3(2H)-isoquinolones from readily available 3-en-5-ynyl nitrones and o-alkynylphenyl nitrones; the reaction mechanism is proposed to involve iminyl ketene species through an oxygen transfer process.

Chem. Commun., 2009, 5233-5235
https://doi.org/10.1039/B910773H
Intramolecular cross-coupling of gem-dibromoolefins: a mild approach to 2-bromo benzofused heterocycles
Highly useful 2-halogenated benzofurans and benzothiophenes can be prepared from readily available gem-dibromoolefins using a mild, ligand-free copper catalyzed cross-coupling procedure.
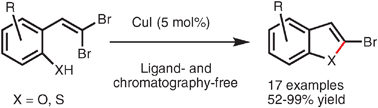
Chem. Commun., 2009, 5236-5238
https://doi.org/10.1039/B912093A
Synthesis of highly enantiomerically enriched planar chiral ruthenium complexes viaPd-catalysed asymmetric hydrogenolysis
Key elements reported are a very efficient microwave synthesis of [RuCp(naphthalene)][PF6], the precursor of [RuCp(CH3CN)3][PF6], and the application of Pd-catalysed asymmetric hydrogenolysis to prochiral ruthenium complexes.
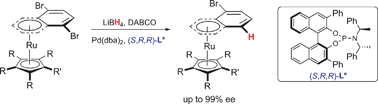
Chem. Commun., 2009, 5227-5229
https://doi.org/10.1039/B910977C
One-step formation of fused tetracyclic skeletons from cyclohexene -diynes and carbon monoxide through Rh(I)-catalyzed [2 + 2 + 2 + 1] cycloaddition reaction
A novel approach to the rapid construction of 5-7-6-5 fused tetracyclic carbocycles and heterocycles from cyclohexene-diynes and CO in just one step through Rh(I)-catalyzed [2 + 2 + 2 + 1] cycloaddition is described.
![Graphical abstract: One-step formation of fused tetracyclic skeletons from cyclohexene-diynes and carbon monoxide through Rh(i)-catalyzed [2 + 2 + 2 + 1] cycloaddition reaction](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=B909781C&imageInfo.ImageIdentifier.Year=2009)
Chem. Commun., 2009, 4569-4571
https://doi.org/10.1039/B909781C
Catalytic amide formation with α′-hydroxyenones as acylating reagents
Amide formation catalyzed by the combination of an N-heterocyclic carbene and 1,2,4-triazole is achieved using α′-hydroxyenones as acylating reagents. No stoichiometric coupling reagents are required.
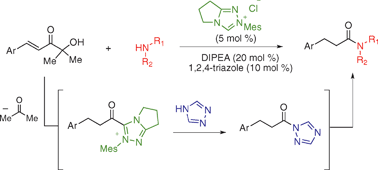
Chem. Commun., 2009, 4566-4568
https://doi.org/10.1039/B909360E
Phosphabarrelene-modified Rh-catalysts: a new and selective route towards hydroxy-functionalized bicyclic imidazoles via tandem reactions
8-Hydroxy-6-methyl-5,6,7,8-tetrahydroimidazo[1,2-a]pyridine is formed selectively in high yields by a one-pot tandem hydroformylation–cyclization sequence.
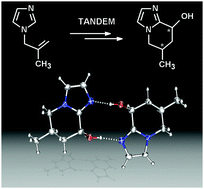
Chem. Commun., 2009, 4944-4946
https://doi.org/10.1039/B911612E
A catalytic synthesis of selectively substituted biaryls through sequential intermolecular coupling involving arene and ketone C–H bond functionalization
Selectively substituted biaryls containing a ketone unit are obtained under mild conditions in satisfactory yields through sequential intermolecular coupling involving ortho-substituted iodoarenes and ketones in the presence of palladium and norbornene as the only catalysts.
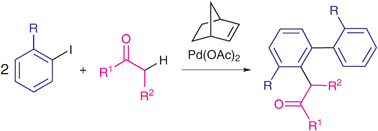
Chem. Commun., 2009, 4892-4894
https://doi.org/10.1039/B909207B
Electronic and steric tuning of chiral diene ligands for rhodium-catalyzed asymmetric arylation of imines
High efficiency was achieved by the use of an electronically and sterically-modified chiral diene ligand in rhodium-catalyzed asymmetric addition reactions. Asymmetric arylation of imines gave the corresponding diarylmethylamines in high yields with high enantioselectivity in the presence of 0.3 mol% of the diene–rhodium catalyst.
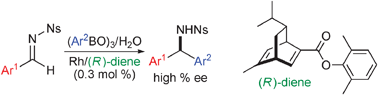
Chem. Commun., 2009, 4815-4817
https://doi.org/10.1039/B904624K
Rhodium catalyzed enantioselective cyclization of substituted imidazoles via C–H bond activation
The enantioselective intramolecular alkylation of substituted imidazoles with enantiomeric excesses up to 98% has been accomplished by rhodium catalyzed C–H bond functionalization with (S,S′,R,R′)TangPhos as the chiral ligand.
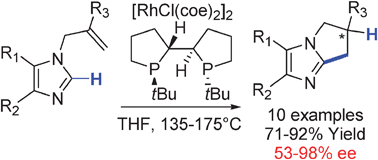
Chem. Commun., 2009, 3910-3912
https://doi.org/10.1039/B902878A
Asymmetric autocatalysis induced by meteoritic amino acids with hydrogen isotope chirality
Achiral meteoritic glycine and α-methylalanine, with hydrogen isotope (D/H) chirality, acted as the source of chirality in asymmetric autocatalysis with amplification of ee to afford highly enantioenriched 5-pyrimidyl alkanols.
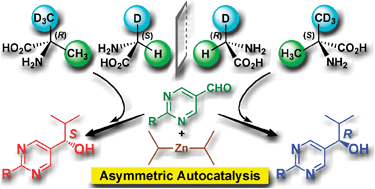
Chem. Commun., 2009, 4396-4398
https://doi.org/10.1039/B908754K
Dynamic kinetic asymmetric transformation in copper catalyzed allylic alkylation
The first dynamic kinetic asymmetric transformation in copper catalyzed allylic alkylation is reported, with enantioselectivities up to 92%.
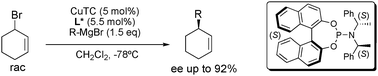
Chem. Commun., 2009, 3868-3870
https://doi.org/10.1039/B907722G
Nickel/AlMe2Cl-catalysed carbocyanation of alkynes using arylacetonitriles
Nickel/Lewis acid dual catalysis was found to effect the carbocyanation reaction of alkynes using arylacetonitriles, giving a range of triply substituted acrylonitriles.
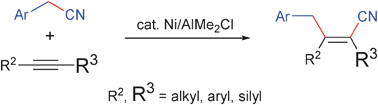
Chem. Commun., 2009, 3931-3933
https://doi.org/10.1039/B907290J
Comparative catalytic C–H vs. C–Si activation of arenes with Pd complexes directed by urea or amide groups
Analysis of regiocontrol in Pd-catalysed C–H activation leads to observations of aryltrimethylsilyl activation and to superior results with urea-based substrates.
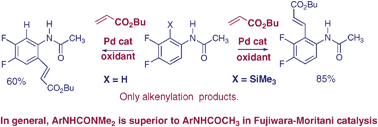
Chem. Commun., 2009, 3874-3876
https://doi.org/10.1039/B905717J
Highly asymmetric cobalt-catalyzed aziridination of alkenes with trichloroethoxysulfonyl azide (TcesN3)
[Co(2,6-DiMeO-ZhuPhyrin)] can aziridinate both aromatic and aliphatic olefins under mild conditions in high yields and excellent enantioselectivities. The catalyst can be conveniently recycled and reused through a precipitation/filtration protocol.
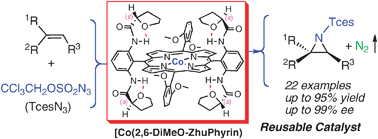
Chem. Commun., 2009, 4266-4268
https://doi.org/10.1039/B905727G
Efficient synthesis of 2,3-dihydro-1H-pyrazoles via a highly selective Pd(0)-catalyzed coupling-cyclization reaction of terminal 2-substituted 2,3-allenyl hydrazines with aryl iodides
2,3-Dihydro-1H-pyrazoles may be highly selectively synthesized via the Pd(0)-catalyzed coupling-cyclization reaction of 4-non-substituted 2-substituted 2,3-allenyl hydrazines with aryl iodides in moderate to good yields.
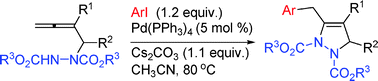
Chem. Commun., 2009, 4263-4265
https://doi.org/10.1039/B903634B
Combination of a binaphthol-derived phosphite and a C1-symmetric phosphinamine generates heteroleptic catalysts in Rh- and Pd-mediated reactions
A heteroleptic catalyst is active in Rh- and Pd-mediated reactions using a 1 : 1 binaphthol-derived phosphite : C1-symmetric phosphinamine ligand combination.
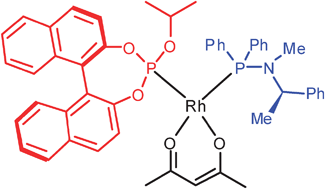
Chem. Commun., 2009, 3539-3541
https://doi.org/10.1039/B908167D
TfOH -catalyzed intramolecular alkyne –ketone metathesis leading to highly substituted five-membered cyclic enones
TfOH-catalyzed intramolecular carbocyclization of tethered alkynyl ketones in MeOH afforded a variety of highly substituted five-membered cyclic enones in good to excellent yields with high selectivities.
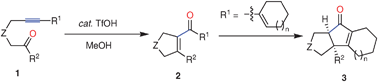
Chem. Commun., 2009, 3533-3535
https://doi.org/10.1039/B905954G
Rhodium-catalyzed asymmetric hydroalkoxylation and hydrosulfenylation of diphenylphosphinylallenes
The rhodium-catalyzed intermolecular asymmetric hydroalkoxylation and hydrosulfenylation of diphenylphosphinylallenes gave chiral allylic phosphine oxides substituted with vinyl ether and thioether moieties in high yields with high enantioselectivities.
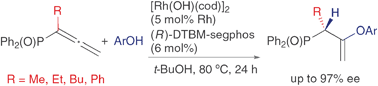
Chem. Commun., 2009, 3528-3530
https://doi.org/10.1039/B900976K
Efficient copper-catalyzed coupling of aryl chlorides, bromides and iodides with aqueous ammonia
Carbon–nitrogen bond formation with a wide range of aryl halides and aqueous ammonia in the presence of catalytic amounts of cuprous oxide has been achieved. The reaction proceeds in aqueous solution and it eliminates the need for inert atmosphere, expensive catalysts and ligands, anhydrous solvents, and base or other additives.
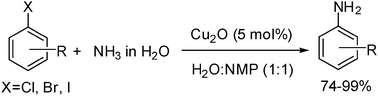
Chem. Commun., 2009, 3035-3037
https://doi.org/10.1039/B904188E
First iron-catalyzed synthesis of oximes from styrenes
Aryl-substituted olefins react with t-butyl nitrite and sodium borohydride in the presence of iron(II)phthalocyanine to give oximes in moderate to high yields.
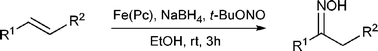
Chem. Commun., 2009, 1990-1992
https://doi.org/10.1039/B900326F
About this collection
This web-based theme issue showcases high quality applications of catalytic reactions to organic synthesis. The collated, invited, and peer-reviewed ChemComm articles highlight cutting edge contributions by international leaders.
The editors of this theme issue are excited by the overwhelming response that the letter of invitation produced and they express their sincere thanks to the authors. The broad spectrum of articles focussing on current developments and frontiers in catalysis for organic synthesis is a celebration of achievements and offers future perspectives in this exciting field of research.
Articles in this web themed issue will be added to this list as soon as they are published. Please return to this page frequently and see it GROW