Efficient inhibition of human papillomavirus 16 L1 pentamer formation by a carboxylatopillarene and a p-sulfonatocalixarene†
Abstract
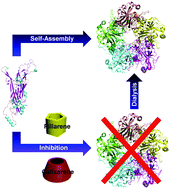
Pillarenes and calixarenes showed obvious inhibition of HPV16 L1 pentamer formation via their selective binding to Arg and Lys residues at the monomer interface, which was reversible after the release of cyclic arenes. Pillarenes are more effective than calixarenes in terms of the inhibition efficiency, attributing to the different kinetics and binding affinity.

 Please wait while we load your content...
Please wait while we load your content...