Zn-substituted heteropoly acids as efficient catalysts for the addition–esterification of 1-hexene†
Abstract
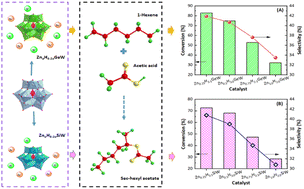
Synthesis of organic esters using olefins as feedstock not only avoids some disadvantages caused by the esterification of alcohols and carboxylic acids but also has important significance for the high-value utilization of olefins. In this study, Zn-substituted germanium tungstic acid (ZnaH4−2aGeW, a = 0.25, 0.5, 0.75, and 1.0) and silicotungstic acid (ZnaH4−2aSiW, a = 0.25, 0.5, 0.75, and 1.0) catalysts were prepared and used to catalyze the addition–esterification of 1-hexene to synthesize sec-hexyl acetate. The physicochemical properties of the catalysts were analyzed using various characterization techniques. The results indicate that Zn0.25H3.5GeW and Zn0.25H3.5SiW have excellent catalytic activity in the series of Zn-substituted germanium tungstic acid and silicotungstic acid salt catalysts, respectively. Moreover, the catalytic activity of Zn0.25H3.5GeW is higher than that of Zn0.25H3.5SiW due to its higher Brønsted acidity. When the conversion of 1-hexene catalyzed by Zn0.25H3.5GeW and Zn0.25H3.5SiW is basically the same by controlling reaction conditions, it was found that the selectivity of sec-hexyl acetate was closely related to the adsorption capacity of the catalyst for acetic acid molecules. Under the conditions of 90 °C, 12 h, 0.4 g catalysts, and a reactant molar ratio of 2.0, the 1-hexene conversion and sec-hexyl acetate selectivity using Zn0.25H3.5GeW as the catalyst are 97.4% and 50.3%, respectively.


 Please wait while we load your content...
Please wait while we load your content...