Enhancing sensitivity in miniature mass spectrometry analysis via dicationic ionic liquid-based matrix-assisted ionization and charge inversion reactions†
Abstract
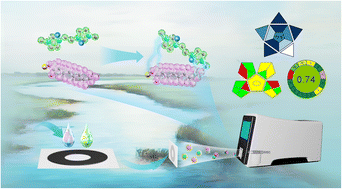
Developing green analytical approaches has become a crucial trend in green chemistry. These approaches encourage direct analysis to avoid any sample treatment that involves energy and reagent consumption or the generation of waste. In this study, a green sensitive analytical workflow was developed for the direct analysis of anionic compounds via a charge inversion strategy using matrix-assisted ionization and a miniature mass spectrometer. Per- and polyfluoroalkyl substances (PFAS), which are a representative category of emerging pollutants, were selected as model analytes for method development. An imidazolium-based germinal dicationic ionic liquid (DIL), 1,1-bis(3-methylimidazolium-1-yl) butylene difluoride ([C4(MIM)2]F2), was chosen as a charge donor to form positively charged complexes with the PFAS for mass spectrometry (MS) analysis. Without any sample treatment, 1 μL of sample was loaded on a paper strip, followed by the addition of a mixture of 3-nitrobenzonitrile and [C4(MIM)2]F2. The adduct complexes were analyzed using a miniature mass spectrometer with tandem MS capability. The signal intensity was enhanced by up to two orders of magnitude in positive ion mode compared to negative ion mode without a DIL. The developed protocol was validated and satisfactory sensitivity was obtained, with limits of detection and quantitation ranging from 5 to 10 μg L−1. Recoveries ranging from 86.6% to 106.3% were achieved, with relative standard deviations between 3.5% and 9.3%. As indicated by three typical green analytical chemistry metric tools, this analytical procedure had a high degree of greenness. The developed protocol was applied to the analysis of real environmental and municipal water samples, demonstrating good potential for the rapid on-site analysis of anionic compounds.


 Please wait while we load your content...
Please wait while we load your content...