A pillar-layered Ni2P–Ni5P4–CoP array derived from a metal–organic framework as a bifunctional catalyst for efficient overall water splitting†
Abstract
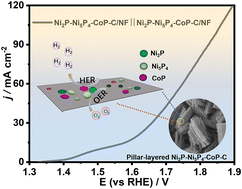
Interfacial engineering emerges as a potent strategy for regulating the catalytic reactivity of metal phosphides. Developing a facile and cost-effective method to construct bifunctional metal phosphides for highly efficient electrochemical overall water splitting remains an essential and challenging issue. Here, a multiphase transition metal phosphide is constructed through the direct phosphorization of a Ni–Co metal–organic framework grown on nickel foam (Ni–Co-MOF/NF), which is prepared by utilizing nickel foam as conductive substrate and nickel source. The resulting transition metal phosphide manifests a pillar-layered morphology, wherein CoP, Ni2P, and Ni5P4 nanoparticles are embedded within each carbon sheet and these carbon sheets assemble into a pillar-shaped structure on the nickel foam (Ni2P–Ni5P4–CoP–C/NF). The heterogeneous Ni2P-Ni5P4–CoP–C/NF with multiple interfaces serves as a highly efficient bifunctional electrocatalyst with overpotentials of −100 mV and 293 mV in the hydrogen evolution reaction and oxygen evolution reaction, respectively, at 50 mA cm−2 in alkaline media. This superior catalytic performance should mainly be ascribed to its enriched active centers and multiphase synergy. When directly applied for alkaline overall water splitting, the Ni2P–Ni5P4–CoP–C/NF couple demonstrates satisfactory activity (1.55 V @10 mA cm−2) along with sustained durability over 18 hours. This method brings fresh enlightenment to the economical and controllable preparation of multi-metal phosphides for energy conversion.


 Please wait while we load your content...
Please wait while we load your content...