Solubility- and melting-based approaches to evaluate the inner-crystal enantiophobia and enantiophilia undergoing a structural change within a homologous series, exemplified by chiral glycerol ethers†
Abstract
For a series of chiral para-alkylphenyl glycerol ethers—para-Alk–C6H4–OCH2CH(OH)CH2(OH) [Alk = Me (1), Et (2), n-Pr (3), n-Bu (4), iso-Pr (5), tert-Bu (6)]—we used information on their solubility in cyclohexane and thermochemical melting parameters to calculate the standard free energies of dissolution, 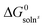 , and melting, ΔG0f. Our observations showed consistent patterns in these parameters within the homologous series. This analysis allowed us to compare the energy characteristics of the crystal structures, thus enabling the quantification of enantiophobic and enantiophilic effects. Furthermore, it facilitated the examination of trends in chiral discrimination as the molecular constitution changed sequentially. We identified distinct trends for enantiopure substances and corresponding racemic compounds within the series studied. In particular, the difference in crystal packing energies among the series members following these trends favours racemic molecular compounds by approximately 2.5 kJ mol−1. However, for (R)-3 and (R)-4, the realised crystal structures are more energetically favoured than expected, thus deviating from the established correlations. This anomaly allows homochiral crystal structure 3 to bridge the energy gap and crystallise from the racemic mixture as a thermodynamically more favourable conglomerate of enantiomeric crystals. In contrast, for racemic compound rac-5, the energy of its molecular packing is lower than expected from the correlations but insufficient to make conglomerate 5 thermodynamically more favourable. At the same time, the formation of racemic compound rac-6 was abnormally preferable in terms of energy. In addition, we presented the X-ray diffraction data for the homo- and heterochiral crystals of 5, thereby complementing the structural information available for the “chiral pool” class of glycerol derivatives.
, and melting, ΔG0f. Our observations showed consistent patterns in these parameters within the homologous series. This analysis allowed us to compare the energy characteristics of the crystal structures, thus enabling the quantification of enantiophobic and enantiophilic effects. Furthermore, it facilitated the examination of trends in chiral discrimination as the molecular constitution changed sequentially. We identified distinct trends for enantiopure substances and corresponding racemic compounds within the series studied. In particular, the difference in crystal packing energies among the series members following these trends favours racemic molecular compounds by approximately 2.5 kJ mol−1. However, for (R)-3 and (R)-4, the realised crystal structures are more energetically favoured than expected, thus deviating from the established correlations. This anomaly allows homochiral crystal structure 3 to bridge the energy gap and crystallise from the racemic mixture as a thermodynamically more favourable conglomerate of enantiomeric crystals. In contrast, for racemic compound rac-5, the energy of its molecular packing is lower than expected from the correlations but insufficient to make conglomerate 5 thermodynamically more favourable. At the same time, the formation of racemic compound rac-6 was abnormally preferable in terms of energy. In addition, we presented the X-ray diffraction data for the homo- and heterochiral crystals of 5, thereby complementing the structural information available for the “chiral pool” class of glycerol derivatives.



 Please wait while we load your content...
Please wait while we load your content...