Homoleptic purine-based NHC iridium(iii) complexes for blue OLED application: impact of isomerism on photophysical properties†
Abstract
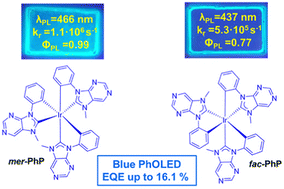
With a motivation to develop deep-blue emitters for organic light-emitting diodes (OLEDs) stereoisomers of a homoleptic N-heterocyclic carbene (NHC) iridium(III) complex bearing 7,9-dihydro-8H-purin-8-ylidene ligands (mer-PhP and fac-PhP) have been synthesized and their photophysical, electrical and electroluminescent properties investigated. The studied complexes only transport holes with the highest hole mobility value of 2 × 10−4 cm2 V−1 s−1 at an electric field of 4.9 × 105 V cm−1 in the case of fac-PhP. When molecularly dispersed in PMMA films, the compounds show bright blue phosphorescence (466 and 437 nm) with photoluminescence quantum yield (ΦPL) values of 0.99 and 0.77. The complexes also possess exceptional thermal stability with a 5% mass loss point at up to 480 °C. While the mer-isomer shows more efficient photoluminescence and a twofold higher radiative rate, it is more sensitive to external stimulus: its ΦPL significantly drops and an emission redshift takes place upon transfer from a rigid to liquid surrounding medium or under exposure to a temperature increase. DFT calculations relate this effect to a weakened metal–ligand bonding at the lowest energy excited triplet state (T1) of the compound, which promotes the extent of non-radiative relaxation processes. On the basis of mer-PhP an efficient blue OLED was prepared with an emission maximum wavelength of 467 nm and a maximum external quantum efficiency of 16.1%.


 Please wait while we load your content...
Please wait while we load your content...