Complex defect chemistry of hydrothermally-synthesized Nb-substituted β′-LiVOPO4†
Abstract
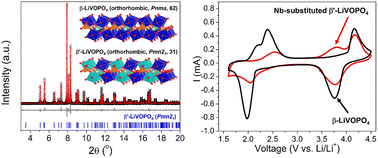
Lithium vanadyl phosphate (LiVOPO4) is a next-generation multielectron battery cathode that can intercalate up to two Li-ions per V-ion through the redox couples of V4+/V3+ and V5+/V4+. However, its rate capacity is undermined by the sluggish Li-ion diffusion in the high-voltage region (4 V for V5+/V4+ redox). Nb substitution was used to expand the crystal lattice to facilitate Li-ion diffusion. In this work, Nb substitution was achieved via hydrothermal synthesis, which resulted in a new, lower symmetry β′-LiVOPO4 phase with preferential Nb occupation of one of the two V sites. This phase presents complex defect chemistries, including cation vacancies and hydrogen interstitials, characterized by a combination of X-ray and neutron diffraction, elemental and thermogravimetric analyses, X-ray absorption spectroscopy, and magnetic susceptibility measurements. The Nb-substituted samples demonstrated improved capacity retention and rate capabilities in the high-voltage region, albeit an enlarged voltage hysteresis related to a partial V4+/V3+ redox reaction, as evidenced by ex situ X-ray absorption spectroscopy and pair distribution function analysis. This work highlights the importance of understanding the complex defect chemistry and its consequence on electrochemistry in polyanionic intercalation compounds.
- This article is part of the themed collection: #MyFirstJMCA


 Please wait while we load your content...
Please wait while we load your content...
