Improved alkaline water electrolysis system for green energy: sulfonamide antibiotic-assisted anodic oxidation integrated with hydrogen generation †
Abstract
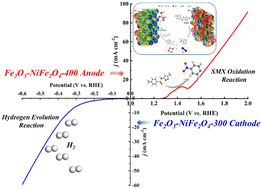
To couple the treatment of antibiotic-polluted wastewater with hydrogen generation, an electrochemical system was built to combine the anodic sulfamethoxazole (SMX) degradation reaction with the cathodic hydrogen evolution reaction (HER). This system showed an excellent pollutant removal efficiency (92.99% ± 3.5%) and H2 yield with only a cell voltage of 2.37 V at 100 mA cm−2. This is attributed to a synergistic effect of indirect oxidation through Fe(VI) and direct oxidation, accounting for approximately 30% and 60% of the total removal, respectively. To further reveal the formation of Fe(VI), the electrostatic potential was analyzed based on density functional theory (DFT). A possible SMX degradation pathway was proposed on the basis of detected intermediates via LC-MS and Quantum chemistry calculations. We utilized the SMX oxidation reaction to replace the oxygen evolution reaction (OER). This work provides an idea for designing a potential system integrated with wastewater treatment and renewable energy production.


 Please wait while we load your content...
Please wait while we load your content...