Scalable synthesis of highly stable cyclopropene building blocks: application for bioorthogonal ligation with tetrazines†
Abstract
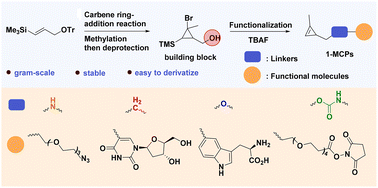
Cyclopropenes are small and highly reactive compounds; however, their synthesis poses significant challenges. This study presents a novel synthetic route for producing a stable and versatile cyclopropene precursor. This approach effectively addresses the issues related to instability, low boiling points, and difficulties in scale-up processes. Additionally, the cyclopropene precursor enabled the easy synthesis of various cyclopropenes with different functional groups and handles. Among the synthesized cyclopropene derivatives, the 1-methylcyclopropene uracil nucleoside derivative 40 had the highest second-order kinetic constant when reacting with the 5-TAMRA-Tz fluorescent dye. Specifically, the kinetic constant reached a value of 56.6 ± 1.94 M−1 s−1 when measured in pure phosphate-buffered saline solution.


 Please wait while we load your content...
Please wait while we load your content...