Hydrodeoxygenation of guaiacol to phenol using endogenous hydrogen induced by chemo-splitting of water over a versatile nano-porous Ni catalyst†
Abstract
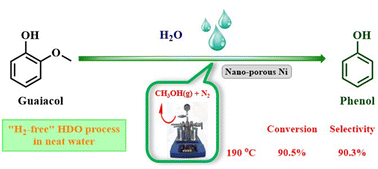
In this work, an innovative route for upgrading biomass-derived phenolic monomers by “hydrogen-free” hydrodeoxygenation (HDO) was proposed and evaluated. The HDO process was integrated with the activation of water and aqueous phase reforming of in situ generated methanol over a nano-porous Ni catalyst and finally the one-pot approach was established achieving high selectivity of bio-phenol. DFT calculations confirmed the crucial role of the Ni catalyst in the activation of water and the following HDO process. The study of the reaction pathway and the mechanism showed that the initial hydrogen source came from water splitting on the surface of the Ni catalyst, which triggered the fracture of the aromatic ether bond to afford phenol and methanol. The subsequent aqueous phase reforming of methanol generated more hydrogen and further accelerated the HDO process. Under the optimized conditions the conversion of guaiacol reached 41.5% and the selectivity of phenol can be 100% at 160 °C. On further increasing the temperature to 190 °C, a high conversion of 96.3% could be achieved while maintaining the selectivity of phenol to 77.9%. After a smart design of methanol release during the reaction, the conversion and selectivity could be further improved to 90.5% and 90.3%, respectively. Overall, the proposed method demonstrates the feasibility of upgrading oxygen-containing biological compounds in a neat water system integrating chemo-splitting of water with using endogenous hydrogen for self-hydrolysis inhibiting external hydrogen supply.


 Please wait while we load your content...
Please wait while we load your content...