Developing post-modified Ce-MOF as a photocatalyst: a detail mechanistic insight into CO2 reduction toward selective C2 product formation†
Abstract
Visible light-driven C–C bond formation to produce C2-based liquid fuel selectively from CO2 is of great interest and remains a challenging task due to uphill electron transfer kinetics. Herein, we have developed [Ru(bpy)2]2+-grafted UiO-66-bpydc Ce-MOFvia post-synthetic modification to harvest visible light based on MLCT (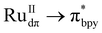 ) transition. The employment of Ru-grafted Ce-MOF facilitates fast electron transfer due to the vacant low-lying 4f orbital of CeIV, which was realized from ultrafast transient absorption (TA) spectroscopy, XANES, and in situ UV-vis spectroscopy. The synergistic effect of facile electron transfer and concomitant accommodation of two CO2 molecules in the proximal defect-site in CeIV leads to facile C–C bond formation via COOH* coupling to yield acetic acid. The catalytic assembly produces 1133 μmol g−1 of acetic acid with an impressive rate of 128 μmol g−1 h−1, suppressing the formation of other C1-based carbonaceous products in water (with selectivity 99.5%, apparent quantum yield (AQY) = 0.93%). A detailed DFT calculation has been performed to understand the mechanistic pathway of C–C bond formation, and the generation of different surface-adsorbed intermediates was further supported by in situ diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy.
) transition. The employment of Ru-grafted Ce-MOF facilitates fast electron transfer due to the vacant low-lying 4f orbital of CeIV, which was realized from ultrafast transient absorption (TA) spectroscopy, XANES, and in situ UV-vis spectroscopy. The synergistic effect of facile electron transfer and concomitant accommodation of two CO2 molecules in the proximal defect-site in CeIV leads to facile C–C bond formation via COOH* coupling to yield acetic acid. The catalytic assembly produces 1133 μmol g−1 of acetic acid with an impressive rate of 128 μmol g−1 h−1, suppressing the formation of other C1-based carbonaceous products in water (with selectivity 99.5%, apparent quantum yield (AQY) = 0.93%). A detailed DFT calculation has been performed to understand the mechanistic pathway of C–C bond formation, and the generation of different surface-adsorbed intermediates was further supported by in situ diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy.



 Please wait while we load your content...
Please wait while we load your content...