P-Centred redox reactions of a 1,4-dihydro-1,4-phosphasiline†
Abstract
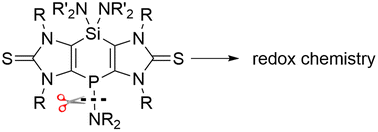
Tricyclic 1,4-dihydro-1,4-phosphasilines 3a,b were synthesized from Si(NR2)2-bridged imidazole-2-thione compounds 2a,b. Based on calculated FMOs of 3b, forecasting a possible P-selective P–N bond cleavage reduction, a redox cycle could be established using solutions of P-centred anionic derivative K[4b]. The cycle started with the oxidation of the latter to give the P–P coupled product 5b which could be chemically reduced by KC8 to yield K[4b], again. All new products have been unambiguously confirmed in solution and solid state.


 Please wait while we load your content...
Please wait while we load your content...