Supramolecular confinement synthesis of ultrafine iron nitride nanocrystals for the oxygen reduction reaction in Zn–air batteries†
Abstract
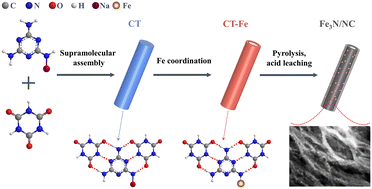
Non-noble metal nitrides have emerged as highly efficient and robust substitutes for platinum-based catalysts toward the oxygen reduction reaction. Herein, we demonstrate a supramolecular confinement strategy to synthesize ultrafine iron nitride (Fe3N, less than 1.5 nm) nanocrystals embedded in a nitrogen-doped carbon matrix, in which the pre-formed Fe–N coordination bonds can anchor the Fe3N nanocrystals and prevent their agglomeration during the pyrolysis process. Reducing the size of Fe3N nanocrystals is conducive to exposing more accessible Fe–N–C ORR active sites and consequently facilitating electrocatalytic ORR activity. The optimized Fe3N/NC-800 exhibits a positive half-wave potential of 0.83 V versus RHE, along with favorable stability and superior tolerance toward methanol. The power density and specific capacity of the optimized Zn–air batteries associated with Fe3N/NC-800 are as high as 90.91 mW cm−2 and 691.59 mA h g−1, respectively. Briefly, a feasible supramolecular confinement strategy is proposed for the construction of ultrafine nanocrystals.


 Please wait while we load your content...
Please wait while we load your content...