Photocatalytic C–F alkylation of trifluoromethyls using o-phosphinophenolate: mechanistic insights and substrate prediction†
Abstract
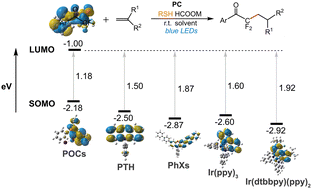
Density functional theory computations reveal the radical mechanism of photocatalytic defluoroalkylation and hydrodefluorination of N-phenyl-2,2,2-trifluoromethylacetamide with o-phosphinophenolate (PO) cooperative catalysis. The energy gaps between the singlet substrate LUMOs and triplet photocatalyst SOMOs can be used as an effective “chemical descriptor” for predicting catalyst activity. Cesium formate assisted C–F bond activation is the most favorable path. A series of available organic structures are computationally predicted as potential substrates.


 Please wait while we load your content...
Please wait while we load your content...