A cascade amplification strategy based on rolling circle amplification and hybridization chain reaction for ultrasensitive detection of pathogens
Abstract
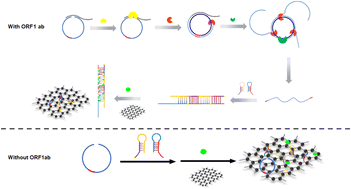
Rapid and accurate detection of a variety of pathogens is very important for the prevention, control, and diagnosis of infectious diseases. Herein, an ultrasensitive nucleic acid isothermal cascade amplification technique based on rolling circle amplification (RCA) coupled with hybridization chain reaction (HCR) was developed for ORF1ab (opening reading frame 1a/b) for SARS-CoV-2 detection. In this scheme, the ORF1ab sequence hybridized with a padlock probe to trigger RCA reaction. Specifically, the recognition site for a unique nicking enzyme was incorporated into the padlock probe to cut the RCA products into short intermediate amplicons, which contain dual HCR initiation sites and can be directly used as primers for HCR. HCR probes, H1 and H2, labeled with FAM (FAM-H1 and FAM-H2) spontaneously participated in the HCR and formed a long nicked dsDNA. Additional probes were quenched by graphene oxide (GO) via π-stacking to decrease the background signal. Meanwhile, the fluorescence signal can be strongly amplified by the synergistic effect of FAM and SYBR green I. The proposed RCA-HCR method can be used to detect ORF1ab at concentrations as low as 7.65 fM. Moreover, the reliability of the RCA-HCR method in serum samples has also been validated. Satisfactory recoveries ranging from 85% to 113% for ORF1ab can be obtained. Therefore, this facile and ultrasensitive RCA-HCR assay provides a new promising tool for ORF1ab analysis and can be extended to the detection of various kinds of pathogens and genetic biomarkers.


 Please wait while we load your content...
Please wait while we load your content...