Modular access to substituted germoles by intramolecular germylzincation†
Abstract
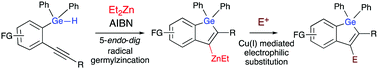
Intramolecular alkyne germylzincation giving access to a wide range of germoles is achieved from triarylhydrogermanes in the presence of diethylzinc and AIBN as radical initiator. The reaction proceeds through activation of the Ge–H bond, leading to a heteroarylzinc intermediate after cyclisation, which can then be involved in a post-functionalisation reaction. Our results show that only 5-endo-dig cyclizations occur, with benzogermoles being exclusively obtained.


 Please wait while we load your content...
Please wait while we load your content...