Ultrasmall Ir nanoclusters on MnO2 nanorods for pH-universal oxygen evolution reactions and rechargeable zinc–air batteries†
Abstract
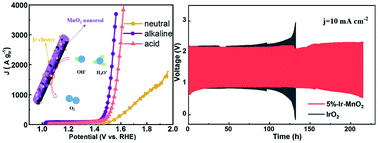
Decreasing the size of noble metals and integrating with transition-metal oxides have been proven as effective means to promote Ir-based catalysts for the oxygen evolution reaction (OER). Herein, we report a facile approach that can uniformly disperse ultrasmall Ir clusters onto MnO2 nanorods for the OER in a universal pH range. Among the x%-Ir-MnO2 (x refers to the mass percentage of Ir) series, 5%-Ir-MnO2 exhibits superior OER performance than other samples, and also exceeds the IrO2 benchmark, as evidenced by small overpotentials of 330 mV (0.2 M PBS, pH = 7.0), 240 mV (1 M KOH, pH = 14.0), and 267 mV (0.5 M H2SO4, pH = 0) to reach a current density of 10 mA cm−2, and markedly superior long-term stability in i–t curves. Furthermore, as an air–cathode catalyst for Zn–air batteries, the 5%-Ir-MnO2 modified battery also demonstrates brilliant performances with large capacities of 655.4 mA h g−1 and 792 mA h g−1, long durability of 120 h and 200 h in alkaline and neutral media, respectively. Such excellent electrocatalytic performances are attributed to the unique structural merits of the ultrasmall Ir clusters, the abundant oxygen vacancies of MnO2 nanorods, and the catalytic synergistic effects between the Ir clusters and the MnO2 substrate.


 Please wait while we load your content...
Please wait while we load your content...