Electrooxidation-induced selective cleavage of C–N bonds of tertiary amines to access thioureas, selenoureas, and 2-aminated benzoselenazoles†
Abstract
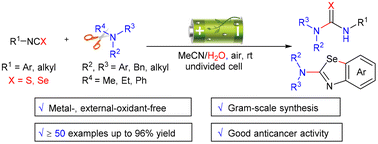
A metal-free, operationally simple, and scalable electrooxidation-induced selective cleavage of C–N bonds of tertiary amines to access asymmetric thiourea, selenourea, and 2-aminated benzoselenazole derivatives is reported. More than 50 examples were reported and were obtained in greater than 96% yield. Importantly, the readily accessible compounds 10c (12.6%, 10.36%) and 24c (6.45% ± 1.40) possess promising anticancer activity against the mouse breast cancer cell line and the human lung adenocarcinoma cancer cell line (A549), reiterating the importance of the developed methodology.


 Please wait while we load your content...
Please wait while we load your content...