Co(iii) 2-ethylhexanoate, a hydrophobic and highly soluble Co(iii) precursor for thin coatings for water electrolysis†
Abstract
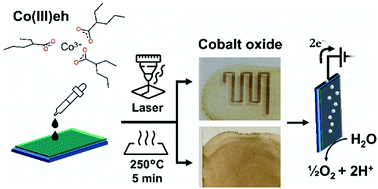
We propose here Co(III) 2-ethylhexanoate (Co(III)eh) as a versatile precursor to make uniform and stable cobalt-containing coatings. Unlike other available salts and complexes of Co(III) that are soluble only in water, polar, or moderately apolar environments, Co(III)eh dissolves easily in a wide spectrum of solvents with dielectric permittivity (ε) ranging from 37.7 (ethanolamine, markedly polar) to 1.9 (hexane, extremely apolar). The high viscosity at room temperature of Co(III)eh can be used to prepare films on a wide variety of substrates. Thin Co(III)eh films can be converted to cobalt oxides at modest temperatures and in a short time (5 min at 250 °C) in an oven, or by laser irradiation (λ = 445 nm). The resulting cobalt oxide films and patterns show state-of-the-art efficiency as electrocatalysts for water electrolysis. These properties make Co(III)eh an ideal precursor for the deposition of cobalt-based coatings for catalysis, energy storage, sensing, and electrochromic applications.


 Please wait while we load your content...
Please wait while we load your content...