Large-Stokes-shifted yellow photoluminescence emission from an imide and polyimides forming multiple intramolecular hydrogen bonds†
Abstract
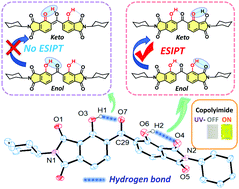
A novel imide compound (DH-MC) and polyimides (DH-PIs) that form multiple intramolecular hydrogen bonds (H-bonds) were synthesised from 2,2′-dihydroxybenzophenone-3,3′,4,4′-tetracarboxylic dianhydride (DHBA) to investigate the effects of distinct H-bond structures on the photoluminescence properties of these compounds. The DHBA moiety, which contains two proton donors and three proton acceptors, can form three types of H-bond structures (MC-0, MC-1, and MC-2). DFT calculations have predicted that the most energetically stable conformation is MC-1, forming an asymmetric H-bond structure, which is consistent with the FT-IR spectroscopy and single-crystal XRD analysis results. A colourless toluene solution of DH-MC exhibited orange fluorescence with a large Stokes shift (ν = 11 905 cm−1), and DH-MC and the DH-PIs exhibited yellow fluorescence with a large ν of >10 000 cm−1 in the solid state, with both originating from excited-state intramolecular proton transfer (ESIPT). In addition, these compounds exhibit small-Stokes-shifted fluorescence from the anionic form of the DHBA moiety, resulting in yellow coloration of the DH-PI film and the DH-MC powder. To reduce coloration, a polyimide copolymer (CoPI) film was prepared using DHBA and 4,4′-oxydiphthalic anhydride (ODPA) in which the molar ratio of DHBA was set at 3%. Owing to the dilution effect and efficient energy transfer from the blue-fluorescent ODPA to the DHBA moiety in the excited state, the colourless and transparent CoPI film exhibited prominent yellow fluorescence with a quantum yield of 0.14. The wavelength-converting spectrum demonstrated that the CoPI film absorbs a wide range of UV radiation from a xenon light source and significantly enhances the yellowish light component via ESIPT emission. The CoPI film is a promising candidate for solar spectral conversion applications.


 Please wait while we load your content...
Please wait while we load your content...