Cationic polymerization of butadiene using alkyl aluminum compounds as co-initiators: an efficient approach toward solid polybutadienes†
Abstract
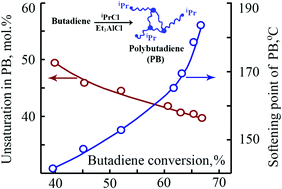
The cationic polymerization of butadiene using secondary alkyl chlorides (isopropyl chloride, 2-chlorobutane) as initiators and alkylaluminum compounds (Et2AlCl, EtAlCl2 and Et3Al) as co-initiators has been investigated. It was shown that secondary alkyl chlorides in conjunction with alkylaluminum compounds are efficient initiating systems for the preparation of fully soluble solid polybutadienes with reduced unsaturation (40–46 mol%) and high glass transition (56–66 °C) and softening (138–184 °C) temperatures. The cationic polymerization of butadiene with iPrCl/Et3Al and iPrCl/Et2AlCl initiating systems is characterized by an induction period which is consistent with the in situ formation of EtAlCl2, a true co-initiator of the polymerization. According to 1D and 2D NMR spectroscopy, the synthesized polybutadienes possessed isopropyl or sec-butyl groups connected with trans-1,4 structures as head groups.


 Please wait while we load your content...
Please wait while we load your content...