Novel cytotoxic 1,10-phenanthroline–triterpenoid amphiphiles with supramolecular characteristics capable of coordinating 64Cu(ii) labels†
Abstract
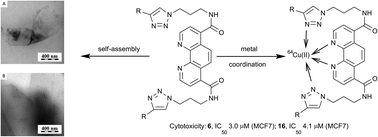
1,10-Phenanthroline was decorated with triterpenoid-based substituents bearing additional spermine units to form amphiphilic molecules. The synthetic procedure designed for the new phenanthroline–triterpenoid amphiphiles is described in detail. Besides 1,10-phenanthroline, all target structures bear 1,4-disubstituted 1,2,3-triazole rings. The target compounds self-assembled into either helical-like or sheet-like nanostructures, depending on the structure of the target molecule, either based on betulinic acid or oleanolic acid, and on the way of binding spermine subunits to the rest of the molecules. They also proved their ability to coordinate 64Cu(II) ions. Finally, the target compounds showed cytotoxicity that was partly dependent on the formation of nanostructures.


 Please wait while we load your content...
Please wait while we load your content...