Photoinduced cytotoxicity of photochromic symmetric diarylethene derivatives: the relation of structure and cytotoxicity†
Abstract
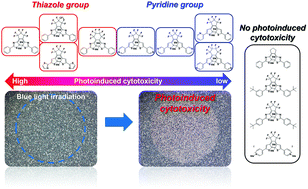
Photopharmacology has been attracting attention for the development of drugs with fewer side effects and lower toxicity by introducing a photoswitch structure in the drug and controlling its spatiotemporal effects by light irradiation. Ideally, to achieve precise spatiotemporal control, it is desirable to use photoresponsive molecules that act as anticancer agents based on molecular switch mechanisms at the molecular level. However, very few reports on photoinduced cytotoxicity have used photoresponsive molecules with simple structures. Here, we investigate the photoinduced cytotoxicity of twelve diarylethene derivatives having thiazole or pyridine rings in their molecules and evaluate them in terms of molecular structure and size. Our results provide insight into molecular design principles for diarylethene with a simple structure toward achieving precise control based on molecular-level switch mechanisms.


 Please wait while we load your content...
Please wait while we load your content...