Preparation of chiral aryl alcohols: a controllable enzymatic strategy via light-driven NAD(P)H regeneration†
Abstract
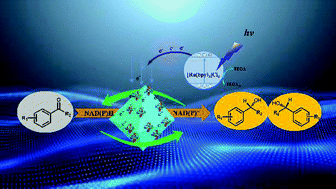
Controllable and mild photoenzymatic production of chiral alcohols was realized by coupling a versatile photochemical NAD(P)H regeneration system with (R)- or (S)-selective ketoreductases. The efficiency of NAD(P)H regeneration was improved using a Rhodium functionalized metal organic framework, namely Rh-UiO-67, to adjust and control electron transport and electron utilization. Furthermore, six different ketoreductases could be successfully immobilized on Rh-UiO-67 and combined with the light-driven NAD(P)H regeneration system to produce chiral aryl alcohols. Various chiral alcohols with complementary (R)- and (S)-conformations can be constructed by this method with high yields (97%) and excellent stereoselectivity (>99% ee).


 Please wait while we load your content...
Please wait while we load your content...