Role of a perhydroxyl radical in the chelator-mediated Fenton reaction†
Abstract
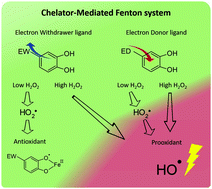
The chelator-mediated Fenton (CMF) chemistry is one of the main biological non-enzymatic systems for oxygen-centered radical generation. A perhydroxyl radical (HO2˙) is a secondary radical specie in CMF systems, however, despite being reported as an iron reducer, its production and role in the CMF redox chemistry are not yet well understood. We assessed the production profiles of HO2˙ and HO˙ generated in situ in CMF systems by EPR, iron-reducing capacity with and without HO2˙, and ligand concentration using CMF systems with p-substituted catecholates between pH values 3.0 and 7.0. The results showed that HO2˙ was generated as a secondary radical in all CMF systems but it also showed evidence of a HO2˙-independent pathway that contributes to the reduction of Fe3+. Along with the current knowledge of the reaction mechanism of the CMF chemistry, the SQ˙ of the ED ligand is proposed to contribute to the generation of HO2˙.


 Please wait while we load your content...
Please wait while we load your content...