Azo dyes containing 1,3,4-thiadiazole fragment: synthesis and properties†
Abstract
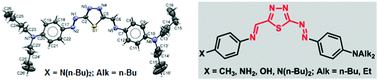
New 1,3,4-thiadiazole derivatives containing a diazenyl group, as well as both a diazenyl and an imino group, were synthesized and their optical and thermal properties were investigated. Initially, new azo compounds containing an azo group directly in the thiadiazole block were obtained by the coupling of diazonium bisulfates based on the previously known 5-derivatives of 2-amino-1,3,4-thiadiazoles with N,N-dialkyl anilines (N(n-Bu)2, NEt2, and NEt(EtOH)). Schiff bases were obtained by the interaction of p-toluidine, p-phenylenediamine and p-aminophenol with azo compounds containing an aldehyde group in the 1,3,4-thiadiazole ring. The new dyes were characterized by spectral (IR, UV, 1H NMR, 13C NMR and mass spectra) and X-ray analyses.


 Please wait while we load your content...
Please wait while we load your content...