An insight into triel bonds in O,O′-diarylphosphorodithioates of thallium(i): experimental and theoretical investigations†
Abstract
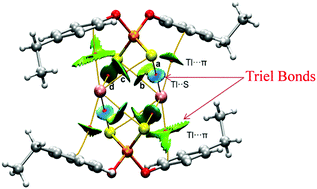
The vital role of triel bonding (TrB) has been highlighted in two new thallium compounds i.e. Tl+[{(4-C2H5)C6H4O}2PS2]2− (1) and Tl[{(4-CH(CH3)2)C6H4O}2PS2]2 (2). These new compounds have been characterized by elemental and spectral analyses. The crystal structures of 1 and 2 belong to the monoclinic and triclinic systems with space groups P21/c and P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , respectively. Interestingly, compound 1 exists in ionic form and is stabilized through intramolecular Tl⋯S (TrBs) interactions wherein the thallium atom is symmetrically positioned in the cleft of the ligand with longer Tl–S distances. In compound 2, the thallium atom is coordinated to one sulfur atom and the other sulfur atom participates in TrB interaction. Both compounds also exhibit intermolecular Tl⋯S, Tl⋯π (TrBs), and C–H⋯Tl (anagostic) interactions, which assist to form unique supramolecular architectures owing to large size as well as coordinative unsaturation on the thallium. These non-covalent TrB interactions were authenticated by Hirshfeld surface analysis (HSA) and density functional theory (DFT) calculations using the quantum theory of atoms-in-molecules (QTAIM) and non-covalent interaction plot (NCI Plot) index methods. TrBs involving Tl have been scarcely described in the literature; therefore this work provides valuable insight into the importance of these unprecedented TrBs in the solid state.
, respectively. Interestingly, compound 1 exists in ionic form and is stabilized through intramolecular Tl⋯S (TrBs) interactions wherein the thallium atom is symmetrically positioned in the cleft of the ligand with longer Tl–S distances. In compound 2, the thallium atom is coordinated to one sulfur atom and the other sulfur atom participates in TrB interaction. Both compounds also exhibit intermolecular Tl⋯S, Tl⋯π (TrBs), and C–H⋯Tl (anagostic) interactions, which assist to form unique supramolecular architectures owing to large size as well as coordinative unsaturation on the thallium. These non-covalent TrB interactions were authenticated by Hirshfeld surface analysis (HSA) and density functional theory (DFT) calculations using the quantum theory of atoms-in-molecules (QTAIM) and non-covalent interaction plot (NCI Plot) index methods. TrBs involving Tl have been scarcely described in the literature; therefore this work provides valuable insight into the importance of these unprecedented TrBs in the solid state.


 Please wait while we load your content...
Please wait while we load your content...