Cinchona alkaloid derivative modified Fe3O4 nanoparticles for enantioselective ring opening of meso-cyclic anhydrides†
Abstract
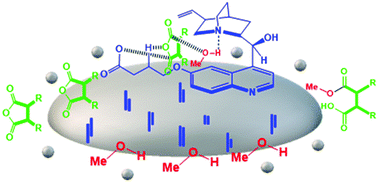
In the present work, the molecular framework of quinidine was modified at the methoxy functional group of the C6′ carbon of the quinoline moiety with a long-chain carboxylic acid group (–COOH) and it was used as a capping agent during the synthesis of Fe3O4 NPs. The resulting modified quinidine capped Fe3O4 (Fe3O4@mQD) NPs were characterized by different techniques and used for enantioselective ring opening (ERO) by methanolysis in a heterogeneous mode of catalysis. The interactions of mQD with the Fe3O4 surface, as well as its % loading, were studied using FTIR spectroscopy and thermal analysis techniques. Reaction parameters like the solvent system, temperature, and substrate were optimized. ERO of succinic and glutaric anhydrides by methanolysis was carried out in the presence of Fe3O4@mQD NPs. The optimized protocol can produce a chiral hemiester with high enantioselectivity (up to 98% ee) and excellent % yield (more than 90% in most of the cases).


 Please wait while we load your content...
Please wait while we load your content...