Rotaxane formation by an allosteric pseudomacrocyclic anion receptor utilising kinetically labile copper(i) coordination properties†
Abstract
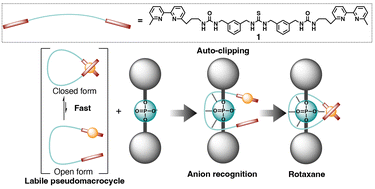
Rotaxanes, which are composed of ring and axle components, are important interlocked molecules with wide applications such as molecular machines and switchable catalysts. The construction of interlocked structures targeting anions is an important issue, as evidenced by the fact that anionic groups are usually abundant in many biomacromolecules. We now report an allosteric pseudomacrocyclic anion receptor as a ring that spontaneously generates a rotaxane in an auto-clipping way, which does not require the successive ring forming reaction like usual clipping, in the presence of an axle with an anionic station. We designed a linear ligand 1 bearing three anion recognition moieties, i.e., one thiourea group at the centre and two urea groups near the 2,2′-bipyridine ends of 1. The complexation of 1 with Cu+ proceeded in an intramolecular manner that quantitatively led to a macrocyclic structure [1·Cu]+. Compared to 1, the anion binding ability of [1·Cu]+ was significantly larger (positive allosteric effect) due to the macrocyclization arrangement of the three anion recognition moieties in a cyclic fashion and electrostatic interaction. In addition, the kinetically labile but thermodynamically stable coordination properties of the pseudomacrocyclic ring unit promoted the spontaneous rotaxane formation with a phosphate axle at room temperature.


 Please wait while we load your content...
Please wait while we load your content...