Light-induced electron transfer/phase migration of a redox mediator for photocatalytic C–C coupling in a biphasic solution†
Abstract
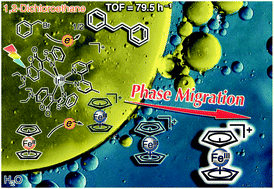
Photocatalytic molecular conversions that lead to value-added chemicals are of considerable interest. To achieve highly efficient photocatalytic reactions, it is equally important as it is challenging to construct systems that enable effective charge separation. Here, we demonstrate that the rational construction of a biphasic solution system with a ferrocenium/ferrocene (Fc+/Fc) redox couple enables efficient photocatalysis by spatial charge separation using the liquid–liquid interface. In a single-phase system, exposure of a 1,2-dichloroethane (DCE) solution containing a Ru(II)- or Ir(III)-based photosensitizer, Fc, and benzyl bromide (Bn-Br) to visible-light irradiation failed to generate any product. However, the photolysis in a H2O/DCE biphasic solution, where the compounds are initially distributed in the DCE phase, facilitated the reductive coupling of Bn-Br to dibenzyl (Bn2) using Fc as an electron donor. The key result of this study is that Fc+, generated by photooxidation of Fc in the DCE phase, migrates to the aqueous phase due to the drastic change in its partition coefficient compared to that of Fc. This liquid–liquid phase migration of the mediator is essential for facilitating the reduction of Bn-Br in the DCE phase as it suppresses backward charge recombination. The co-existence of anions can further modify the driving force of phase migration of Fc+ depending on their hydrophilicity; the best photocatalytic activity was obtained with a turnover frequency of 79.5 h−1 and a quantum efficiency of 0.2% for the formation of Bn2 by adding NBu4+Br− to the biphasic solution. This study showcases a potential approach for rectifying electron transfer with suppressed charge recombination to achieve efficient photocatalysis.


 Please wait while we load your content...
Please wait while we load your content...
