B–O–B bridged BOPPY derivatives: synthesis, structures, and acid-catalyzed cis–trans isomeric interconversion†
Abstract
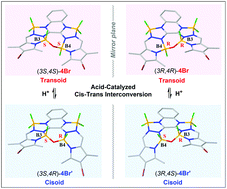
A new class of BOPPY derivatives has been facilely synthesized by a two-step reaction of coupling 3,5-dimethylpyrrole-2-carbaldehyde with 2,3-dihydrazinoquinoxaline (QDH) followed by coordinating with BF3·OEt2. The reaction mainly produces a triboron complex 3 within 1 h, while a pair of B–O–B bridged trans–cis isomers 4 and 4′ are formed as the reaction time elongates to 8 h. Moreover, two bromide products 4Br and 4Br′ are prepared almost quantitatively by the bromination of 4 and 4′, respectively. The coordinated B–N bonds impede the free rotation of the B–O–B bridge, resulting in a high-energy polytopal rearrangement that makes the diastereomers 4, 4′ and 4Br, 4Br′ separable and stable under ambient conditions. Interestingly, these two diastereomeric pairs undergo feasible cis–trans interconversion in the presence of weak acid due to the acid-catalyzed B–N bond cleavage followed by rotational isomerism. In addition, optical, electrochemical and theoretical results suggest that the conformational differences in the (BF)O(BF) part have little effect on the photophysical and electronic properties of such compounds.


 Please wait while we load your content...
Please wait while we load your content...