Ru/Rh catalyzed selective hydrogenation of CO2 to formic acid: a first principles microkinetics analysis†
Abstract
Reverse water gas shift (RWGS, CO formation) and formic acid (FA) production are the two main reaction pathways during the initial hydrogenation stages of CO2 methanation. Here, we predict that unsupported and oxygen deficient TiO2 surface [TiO2(v)] supported Rux/Rhx nanoclusters can efficiently and selectively convert CO2 into FA in comparison with CO. It has been revealed that unsupported Rhx nanoclusters are better catalysts than Rux nanoclusters (x < 7); among them, Rh4 exhibits the highest TOF value of 0.19 s−1 at 450 K and 1 atm, typical experimental conditions for the CO2 reduction reaction. In contrast, a supported Ru4 catalyst exhibits a higher TOF value than Rh4. Both CO2 binding ability and H-migration rates are found to be accelerated by decreasing the size of the metal cluster on the oxide support. A dual atom Rh2 site catalyst is predicted to be a better CO2 hydrogenation catalyst than other larger sized supported clusters under ambient conditions. Microkinetics analyses suggest a very good product selectivity over the Rh2@TiO2(v) catalyst toward FA in comparison with CO. At 450 K, the predicted TOF and EApp are 2.11 × 10−6 s−1 and 1.49 eV, respectively. The reaction passes through a sequential hydrogenation of 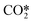 into HCOO* followed by FA*, where desorption of FA* is found to be the rate determining step. However, above 500 K, the rate of FA production becomes faster over Ru4@TiO2(v).
into HCOO* followed by FA*, where desorption of FA* is found to be the rate determining step. However, above 500 K, the rate of FA production becomes faster over Ru4@TiO2(v).



 Please wait while we load your content...
Please wait while we load your content...