Electrochemical promotion of copper nanoparticles for the reverse water gas shift reaction†
Abstract
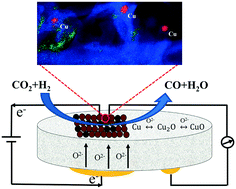
Electrochemical promotion of catalysis (EPOC) is one of the promising ways to in situ control and enhance catalytic processes of the reverse water gas shift reaction (RWGS), which recycles carbon dioxide (CO2) into carbon monoxide (CO). In EPOC, a metal catalyst is deposited on a solid electrolyte that under polarization supplies ions (O2−, H+, Na+, etc.) towards the catalyst surface, leading to the alteration of the catalyst work function, and as a result its catalytic performance. In this study, the catalytic activity and EPOC of copper (Cu) nanoparticles (NPs), an inexpensive metal, are presented and show to be better than those of other non-noble metals, such as iron (Fe) and cobalt (Co). Through characterizations like SEM, STEM and XPS, the change from lower oxidized state to higher oxidized state of copper catalyst before and after reaction is confirmed, and Cu2O and metallic copper are taken as the active states. It is found that the EPOC only occur under positive polarizations and vary significantly under different reaction conditions, indicating that the RWGS reaction over Cu NPs deposited on yttria-stabilized zirconia (YSZ) follows the redox mechanism. The highest electrochemical promotion of Cu NPs occurs under an application of +2 V and CO2 : H2 ratio of 1 : 1 at 400 °C with an enhancement ratio (ρ) of 1.2 and apparent faradaic efficiency (Λ) of 6.52.


 Please wait while we load your content...
Please wait while we load your content...