Modulating catalytic oxygen activation over Pt–TiO2/SiO2 catalysts by defect engineering of a TiO2/SiO2 support†
Abstract
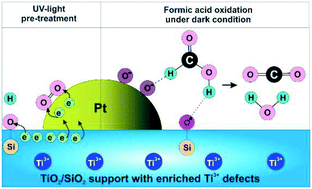
Binary TiO2/SiO2 oxides were synthesized via flame spray pyrolysis as supports for a Pt catalyst. The effect of the mole ratio of the silica on the catalyst characteristics and catalytic performance for formic acid oxidation under dark (non-illuminated) conditions and following UV light pre-treatment was examined. As the TiO2 : SiO2 ratio increased, the following was observed: (i) the specific surface area gradually decreased from ∼325 m2 g−1 to ∼50 m2 g−1; (ii) the crystal structure was transformed from amorphous titania to crystalline anatase phase; (iii) the defect sites comprising Ti3+, E′ centres, and non-bridging oxygen hole centers (NBOHCs) were maximum at a TiO2 : SiO2 ratio of 1 : 2 (Pt/1TiO2–2SiO2); and (iv) oxygen adsorbed on the Pt deposit surface (PtOads) was a maximum for Pt/1TiO2–2SiO2. The catalytic activity of Pt–TiO2/SiO2 was strongly dependent on the TiO2/SiO2 ratio and was further enhanced by hydrogenation and UV light pre-treatment. In line with the greatest presence of support-based defects and PtOads species, the Pt/1TiO2–2SiO2 catalyst exhibited the highest catalytic rate following UV light pre-treatment. The simultaneously boosted presence of PtOads and defect sites (comprising Ti3+, E′ centers, and NBOHC) on the Pt–TiO2/SiO2 catalysts following UV-light pre-treatment is proposed as the origin of the enhanced formic acid oxidation reaction.


 Please wait while we load your content...
Please wait while we load your content...