Vacuum ultraviolet photodissociation of OCS via the 21Σ+ state: the S(1D2) elimination channel
Abstract
The photodissociation of OCS is necessary to model the primary photochemical processes of OCS in the global cycling of sulfur and interstellar photochemistry. Here, by combining the time-sliced velocity-map ion imaging technique with the single vacuum ultraviolet photon ionization method, we have studied the CO(1Σ+, v) + S(1D2) photoproduct channel from the OCS photodissociation via the eight different vibrational resonances (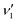 = 1–8) in the 21Σ+(
= 1–8) in the 21Σ+(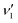 , 1, 0) ← X1Σ+(0, 0, 0) band. From the measured S(1D2) images, the wavelength-dependent CO(1Σ+, v) vibrational state populations have been obtained in the wavelength range of 142.98–154.37 nm. The majority of the CO(1Σ+, v) photoproducts are shown to abruptly populate from low vibrational states to high vibrational states as the photolysis wavelength decreases from 152.38 to 148.92 nm. The anisotropy parameters (β) for the CO(1Σ+, v) + S(1D2) channel have also been determined from the images of the S(1D2) photoproducts. It is found that the vibrational state-specific β-values present a similar decreasing trend with increasing CO vibrational excitation for all the eight vibrational resonances of OCS*(21Σ+). These observations indicate that there is a possibility that more than one non-adiabatic dissociation pathways with different dissociation lifetimes are involved in the formation of CO(1Σ+) + S(1D2) photoproducts from the initial vibronic levels of the 21Σ+ state to the final dissociative state.
, 1, 0) ← X1Σ+(0, 0, 0) band. From the measured S(1D2) images, the wavelength-dependent CO(1Σ+, v) vibrational state populations have been obtained in the wavelength range of 142.98–154.37 nm. The majority of the CO(1Σ+, v) photoproducts are shown to abruptly populate from low vibrational states to high vibrational states as the photolysis wavelength decreases from 152.38 to 148.92 nm. The anisotropy parameters (β) for the CO(1Σ+, v) + S(1D2) channel have also been determined from the images of the S(1D2) photoproducts. It is found that the vibrational state-specific β-values present a similar decreasing trend with increasing CO vibrational excitation for all the eight vibrational resonances of OCS*(21Σ+). These observations indicate that there is a possibility that more than one non-adiabatic dissociation pathways with different dissociation lifetimes are involved in the formation of CO(1Σ+) + S(1D2) photoproducts from the initial vibronic levels of the 21Σ+ state to the final dissociative state.



 Please wait while we load your content...
Please wait while we load your content...