Amorphous calcium carbonate monohydrate containing a defect hydrate network by mechanochemical processing of mono-hydrocalcite using ethanol as auxiliary solvent†
Abstract
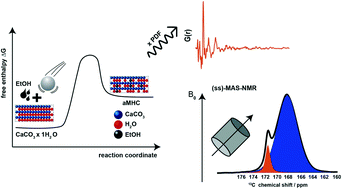
Amorphous calcium carbonate (ACC) is a precursor in the biomineralization of crystalline CaCO3. The lifetime of transient ACC in nature is regulated by biomacromolecules. The relevance of ACC in materials science is related to our understanding of CaCO3 crystallization pathways. Hydrated ACC and calcium carbonate monohydrate (CCM) are similar in their chemical composition, precipitation condition, and thermal behavior. We describe a fast and reliable way to prepare calcium carbonate monohydrate-like ACC (aCCM) in a planetary ball mill using ethanol as auxiliary solvent. Amorphization could not be achieved with apolar auxiliary solvents like cyclohexane. A consistent picture of the amorphization reaction was derived by combining different scattering methods and spectroscopies coupled with thermal analysis. Powder diffraction and total scattering show the absence of long-range order and complete amorphization. Metastable aCCM is stabilized by temporarily and partially displacing structural water with ethanol, which cannot be incorporated into the hydrate network of CCM due to its molecular size. These solvent “defects” in the hydrate network of the CCM structure are crucial to suppress the stabilization of the hydrated CCM polymorph by hydrogen bonding, while foreign ions (∼10% Na+ or HPO42−) are typically required to generate ionic defects in anhydrous CaCO3. NMR and thermal analysis yield an approximate composition CaCO3·0.48H2O for aCCM.


 Please wait while we load your content...
Please wait while we load your content...