Selectivity considerations of host compound trans-9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboxylic acid when presented with pyridine and picoline mixtures: charge-assisted versus classical hydrogen bonding†
Abstract
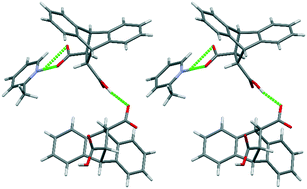
trans-9,10-Dihydro-9,10-ethanoanthracene-11,12-dicarboxylic acid (H1) was assessed for its host behaviour in pyridine (PYR) and 2-, 3- and 4-methylpyridine (2MP, 3MP and 4MP). Each of these guest compounds was enclathrated, and host : guest ratios varied between 1 : 1 and 1 : 2. In binary guest mixtures, H1 was highly selective for 2MP and 3MP when the other guest was either PYR or 4MP, and selectivity coefficients (K) in 2MP/PYR and 2MP/4MP were significant (8.3 and 7.1). SCXRD analyses on the solids recovered from the H1/2MP and H1/3MP recrystallization experiments revealed that these were, in fact, not conventional inclusion compounds but salts: one host carboxylic acid proton was displaced and resided on the guest nitrogen atom. The host preference for 2MP and 3MP was thus not entirely due to guest enclathration but also as a result of the strong charge-assisted hydrogen bonding contributions (proton transfer was not observed in H1/PYR and H1/4MP). Finally, the relative thermal stabilities of all complexes concurred with data from guest/guest competitions: preferred guests always formed complexes with greater stabilities relative to guests less favoured.


 Please wait while we load your content...
Please wait while we load your content...