Dihalo bismuth cations: unusual coordination properties and inverse solvent effects in Lewis acidity†
Abstract
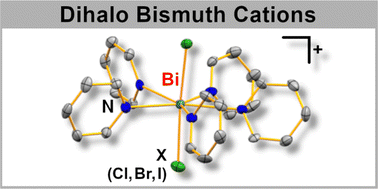
A series of well-defined cationic hepta-coordinate bismuth halides [BiX2(py)5][B(3,5-(CF3)2-C6H3)4] (X = Cl, Br, I), stabilized only by substitutionally labile solvent molecules, were synthesized and fully characterized. Their apparent D5h symmetry with a lone pair at the central atom is unprecedented for main group compounds. The potential of BiX3 to show unexpectedly high Lewis acidities in moderately polar solvents is likely due to the formation of [BiX2(solv)5]+ and related ionic species.


 Please wait while we load your content...
Please wait while we load your content...