Heteroatom-doped carbon-based oxygen reduction electrocatalysts with tailored four-electron and two-electron selectivity
Abstract
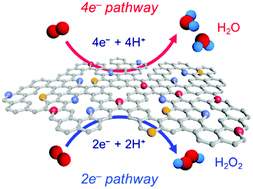
Oxygen reduction reaction (ORR) plays a pivotal role in electrochemical energy conversion and commodity chemical production. Oxygen reduction involving a complete four-electron (4e−) transfer is important for the efficient operation of polymer electrolyte fuel cells, whereas the ORR with a partial 2e− transfer can serve as a versatile method for producing industrially important hydrogen peroxide (H2O2). For both the 4e− and 2e− pathway ORR, platinum-group metals (PGMs) have been materials of prevalent choice owing to their high intrinsic activity, but they are costly and scarce. Hence, the development of highly active and selective non-precious metal catalysts is of crucial importance for advancing electrocatalysis of the ORR. Heteroatom-doped carbon-based electrocatalysts have emerged as promising alternatives to PGM catalysts owing to their appreciable activity, tunable selectivity, and facile preparation. This review provides an overview of the design of heteroatom-doped carbon ORR catalysts with tailored 4e− or 2e− selectivities. We highlight catalyst design strategies that promote 4e− or 2e− ORR activity. We also summarise the major active sites and activity descriptors of the respective ORR pathways and describe the catalyst properties controlling the ORR mechanisms. We conclude the review with a summary and suggestions for future research.
- This article is part of the themed collection: Nanoscale Horizons, Nanoscale, and ChemComm: Nanocatalysis


 Please wait while we load your content...
Please wait while we load your content...