Achieving visible-light-excited organic room-temperature phosphorescence by manipulating p–π conjugation†
Abstract
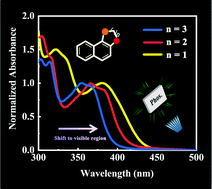
The development of organic room-temperature phosphorescence (RTP) materials has been a rich research field. However, there are still limited practical and directive strategies to regulate the phosphorescence emission. Herein, six naphthalene-based thiochromanone analogs with different heterocycles were synthesized. And all these molecules exhibited bright phosphorescence when embedded into a polyvinyl alcohol matrix under ambient conditions. The absorption spectra showed a distinct red-shift as the size of the heterocycle shrunk, which resulted from the facilitated p–π conjugation. In particular, the absorption spectra of molecules with a five-membered heterocycle were extended to visible light, which is rarely reported for RTP materials. These results might present a new strategy to adjust the photophysical properties of phosphors to obtain phosphorescence materials with the desired excitation and emission wavelength.
- This article is part of the themed collection: Journal of Materials Chemistry C HOT Papers


 Please wait while we load your content...
Please wait while we load your content...