Potential molecular and graphene oxide chelators to dissolve amyloid-β plaques in Alzheimer's disease: a density functional theory study
Abstract
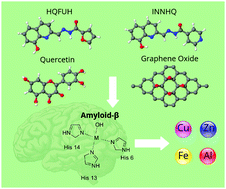
The onset of Alzheimer's disease (AD) is caused by amyloid-β (Aβ) aggregation. Elevated levels of metals, specifically copper, zinc, iron, and aluminum, accumulate in senile Aβ; plaque deposits, disrupting normal brain homeostasis and cognitive functions. In this investigation, we studied the potential of several molecular and graphene oxide chelators to be used for future AD research and chelation therapy. To understand the interactions between selected metals (Cu, Zn, Fe, and Al), the Aβ peptide, and various potential metal chelating compounds, we implemented the density functional theory (DFT) method to calculate the binding energies of each metal–molecule complex. The binding energy of each metal–chelator complex was compared with that of the metal–Aβ compound to determine the chelation potential of the selected chelator. The potential chelating agents studied were 8-hydroxyquinoline-2-carboxaldehyde isonicotinoyl hydrazone (INNHQ), 8-hydroxyquinoline-2-carboxaldehyde 2-furoyl hydrazone (HQFUH), quercetin, and graphene oxide (GO). Our calculated binding energies revealed that the HQFUH molecule holds direct ability to chelate copper, zinc, iron, and aluminum. In addition, the GO complex with a 12.5% oxygen concentration demonstrates aluminum chelation ability. Our results demonstrate that HQFUH and GO can be used in future AD drug development research and therapy to target toxic metal–Aβ interactions and reduce Aβ aggregation.


 Please wait while we load your content...
Please wait while we load your content...