A dramatic reduction in the sintering temperature of the refractory sodium β′′-alumina solid electrolyte via cold sintering†
Abstract
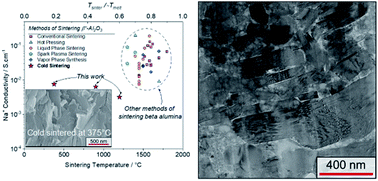
The cold sintering process is successfully applied to one of the most refractory solid-state sodium-ion electrolytes, namely sodium beta alumina (SBA). By using a hydroxide-based transient solvent, SBA is densified below 400 °C, whereas conventional solid-state sintering is known to require sintering temperatures around 1600 °C. This dramatic reduction in sintering temperature (ca. Tsinter ∼ 20% of Tm) is achieved by cold sintering with the addition of 10 wt% solid NaOH transient phase, 360 MPa of uniaxial pressure, and heating to 350–375 °C, for a dwell time of three hours. The resulting pellets exceed 90% of the theoretical density for SBA and exhibit ionic conductivities of ∼10−2 S cm−1 at 300 °C, as measured by electrochemical impedance spectroscopy. The structural changes occurring during cold sintering are reversed with an intermediate temperature annealing step (ca. 1000 °C) which improves the ionic conductivity. This study therefore highlights the opportunities and remaining challenges in applying cold sintering to refractory, air-sensitive, electroceramics.


 Please wait while we load your content...
Please wait while we load your content...
