Unlocking veiled oxygen redox in Na-based earth-abundant binary layered oxide†
Abstract
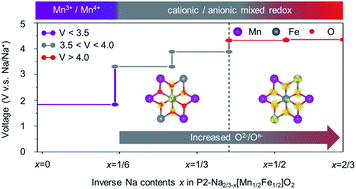
Oxygen-redox reactions (ORRs) in Na-based transition metal (TM) layered oxides have attracted a great deal of attention as a means of overcoming the intrinsic low energy density of the cathodes of sodium-ion batteries (SIBs), because their activities occur above ≈4.0 V versus Na+/Na and deliver extra capacity beyond the TM-based redox contribution. Intriguingly, Mn-based binary-layered oxides (e.g., Na1−x[Mn1−yNiy]O2), have recently been re-examined to elucidate the ORR involvement that had been assigned to the Ni3+/Ni4+ redox couple. Accordingly, Na1−x[Mn1/2Fe1/2]O2, a representative abundant cathode, was reinvestigated in detail using first-principles calculations to unlock veiled ORRs upon charging. First, the charge variations of Mn, Fe, and O showed that a typical Mn3+/Mn4+ cationic redox is a principal factor in charge compensation for 0.25 ≤ x ≤ 0.5 in Na1−x[Mn1/2Fe1/2]O2, whereas a dominant ORR contributes to the charge compensation with the partial Fe redox for a value above x = 0.5. Second, the ORR is selective depending on the oxygen site coordinates with Fe-rich (2Fe–O–Mn) or Mn-rich (Fe–O–2Mn) environments. Third, the Fe–O chemical bonding features reveal that the Jahn–Teller distortion determines the priority of the oxygen redox reaction ≈4.0 V. Along these lines, our findings provide insights into directions in utilizing the veiled oxygen redox reaction of Nax[Mn1−yFey]O2 binary oxides.


 Please wait while we load your content...
Please wait while we load your content...