A straight, open and macro-porous fuel electrode-supported protonic ceramic electrochemical cell†
Abstract
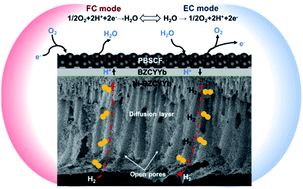
The electrochemical performance of an electrode supported protonic ceramic electrochemical cell (PCEC) often suffers from high mass transport resistances, especially at high current densities. Here, we report our findings in the structure tailoring of the electrode substrate to facilitate mass transport via a modified phase inversion technique, ultimately resulting in lower polarization resistances and higher outputs. A Ni–BaZr0.1Ce0.7Y0.1Yb0.1O3 (BZCYYb) substrate, with straight and open finger-like pores (∼30–80 μm in diameter; ∼500 μm in length), has been successfully fabricated via tape casting and phase inversion of a graphite layer and a substrate layer sequentially. Single cells with such a unique microstructure demonstrate better electrochemical performance when compared with dry-pressed cells under the same conditions. For example, when operated in a fuel cell mode, a peak power density of 1.389 W cm−2 and a polarization resistance of ∼0.039 Ω cm2 at 700 °C (vs. 0.929 W cm−2 and 0.113 Ω cm2 for dry-pressed cells) have been achieved when using hydrogen (with 3% H2O) as the fuel and ambient air as the oxidant. Furthermore, a current density of ∼−2.066 A cm−2 has been achieved at 1.3 V when the cell is operated in an electrolysis mode (3% steam in air) at 700 °C, much higher than that for dry pressed cells (only ∼−1.615 A cm−2 at 1.3 V). Since the air electrode and electrolyte are kept the same, the performance improvement can be attributed to the facilitated mass transport within the electrode substrate. It is concluded that the presence of finger-like pores facilitates the mass transportation in the substrate, thereby reducing the concentration polarization.


 Please wait while we load your content...
Please wait while we load your content...